Abstract
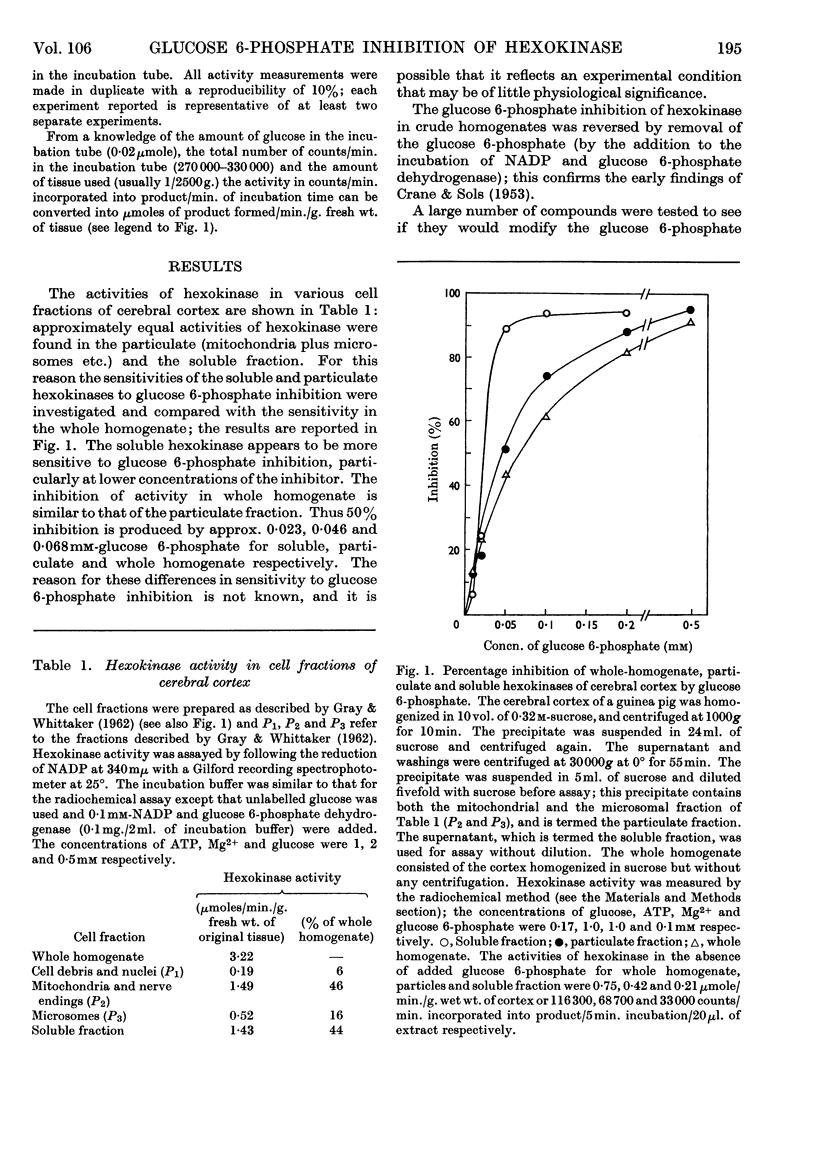
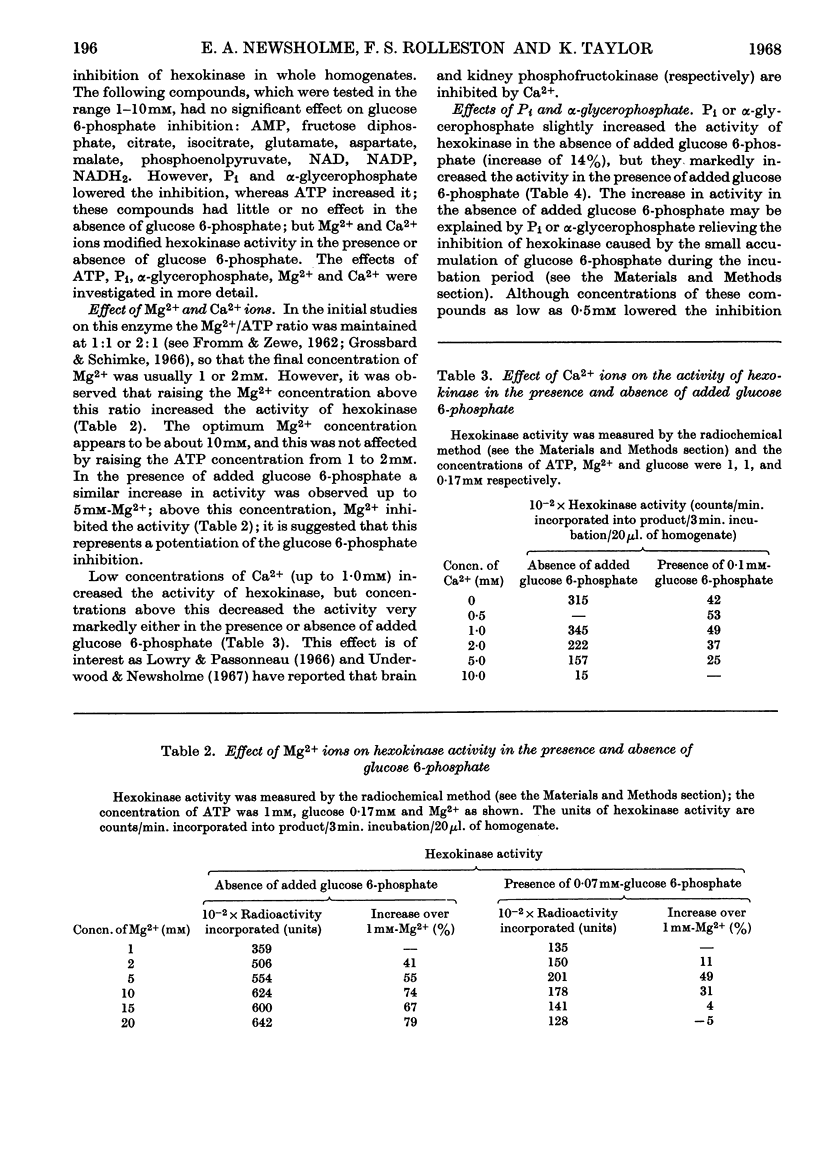
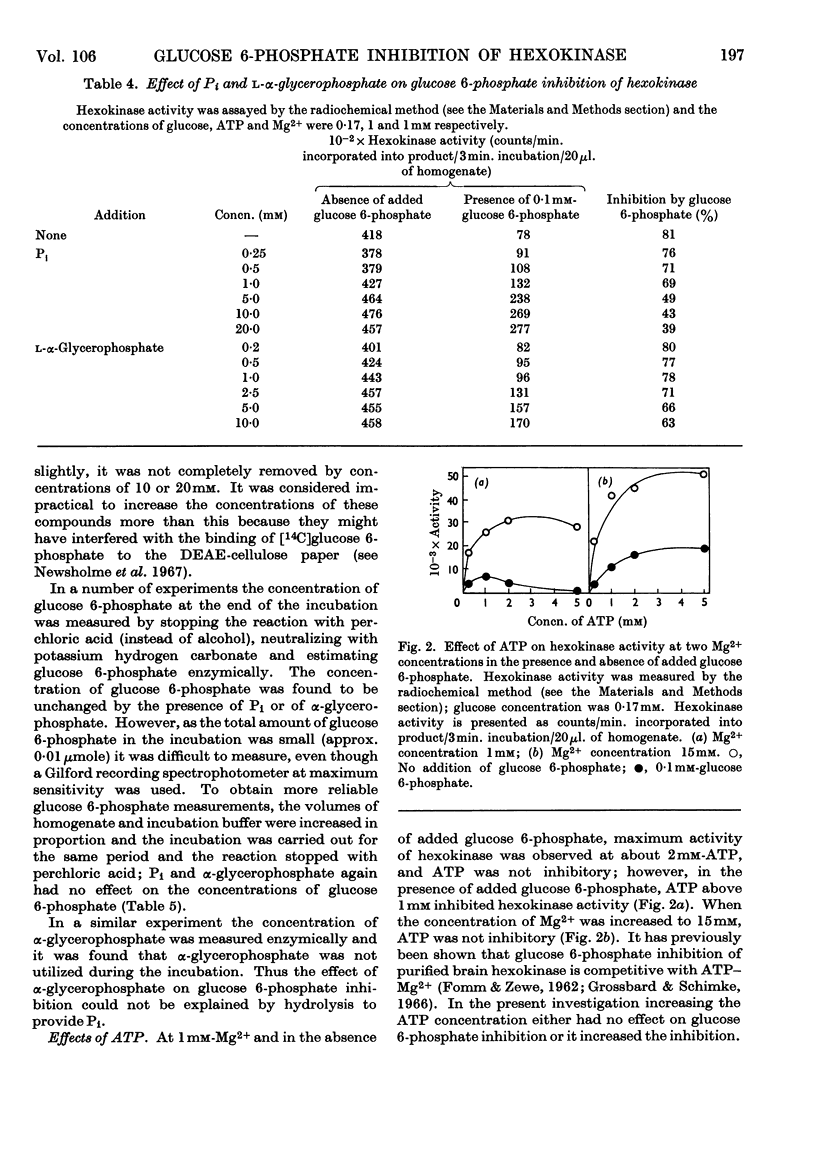
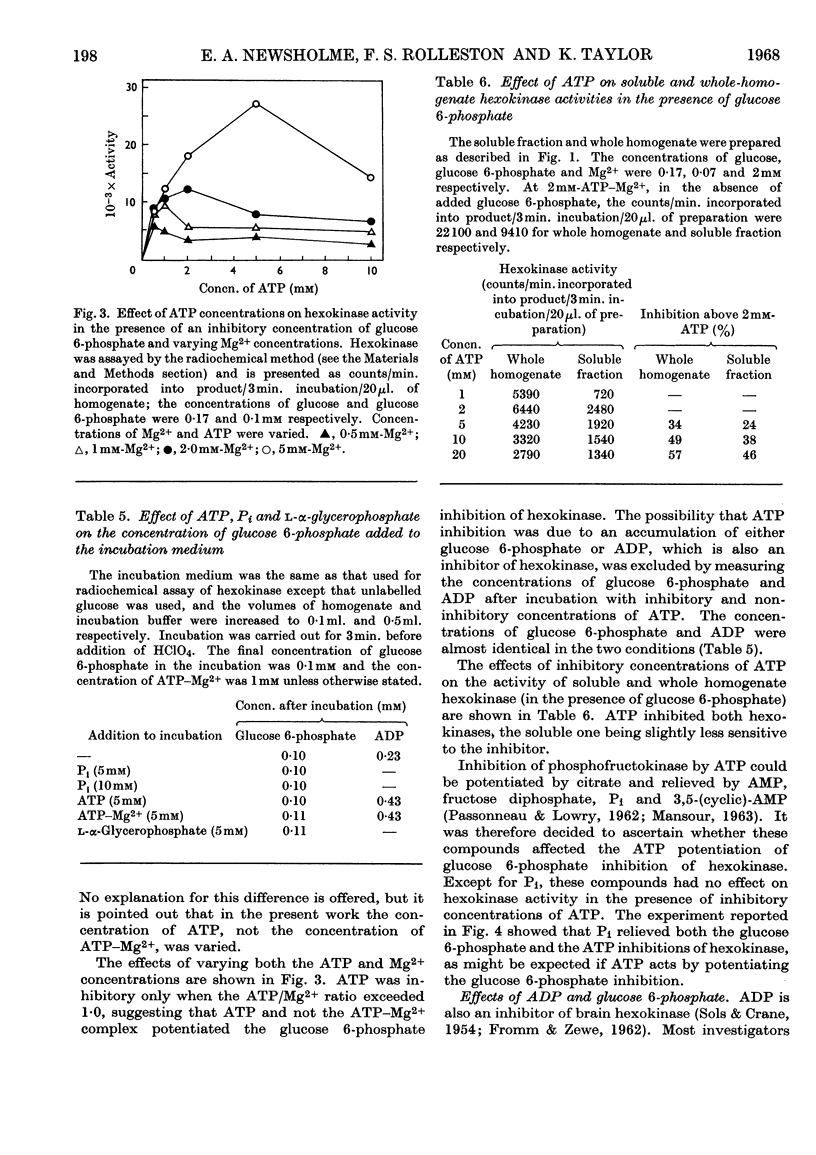
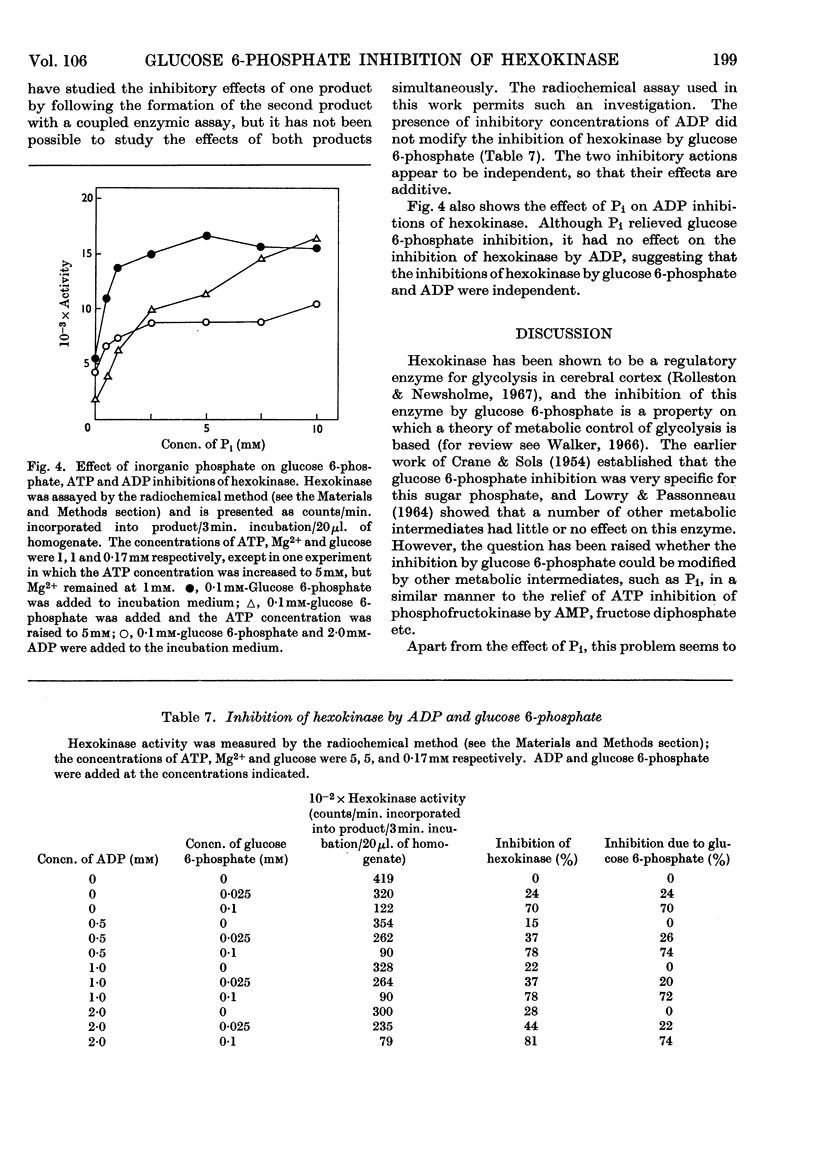
1. The inhibition of hexokinase by glucose 6-phosphate has been investigated in crude homogenates of guinea-pig cerebral cortex by using a sensitive radio-chemical technique for the assay of hexokinase activity. 2. It was observed that 44% of cerebral-cortex hexokinase activity did not sediment with the microsomal or mitochondrial fractions (particulate fraction), and this is termed soluble hexokinase. The sensitivities of soluble and particulate hexokinase, and hexokinase in crude homogenates, to the inhibitory actions of glucose 6-phosphate were measured; 50% inhibition was produced by 0·023, 0·046 and 0·068mm-glucose 6-phosphate for soluble, particulate and crude homogenates respectively. 3. The optimum Mg2+ concentration for the enzyme was about 10mm, and this appeared to be independent of the ATP concentration. In the presence of added glucose 6-phosphate, raising the Mg2+ concentration to 5mm increased the activity of hexokinase, but above this concentration Mg2+ potentiated the glucose 6-phosphate inhibition. When present at a concentration above 1mm, Ca2+ ions inhibited the enzyme in the presence or absence of glucose 6-phosphate. 4. When the ATP/Mg2+ ratio was 1·0 or below, variations in the ATP concentration had no effect on the glucose 6-phosphate inhibition; above this value ATP inhibited hexokinase in the presence of glucose 6-phosphate. ATP had an inhibitory effect on soluble hexokinase similar to that on a whole-homogenate hexokinase, so that the ATP inhibition could not be explained by a conversion of particulate into soluble hexokinase (which is more sensitive to inhibition by glucose 6-phosphate). It is concluded that ATP potentiates glucose 6-phosphate inhibition of cerebral-cortex hexokinase, whereas the ATP–Mg2+ complex has no effect. Inorganic phosphate and l-α-glycerophosphate relieved glucose 6-phosphate inhibition of hexokinase; these effects could not be explained by changes in the concentration of glucose 6-phosphate during the assay. 5. The inhibition of hexokinase by ADP appeared to be independent of the glucose 6-phosphate effect and was not relieved by inorganic phosphate. 6. The physiological significance of the ATP, inorganic phosphate and α-glycerophosphate effects is discussed in relation to the control of glycolysis in cerebral-cortex tissue.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRANE R. K., SOLS A. The association of hexokinase with particulate fractions of brain and other tissue homogenates. J Biol Chem. 1953 Jul;203(1):273–292. [PubMed] [Google Scholar]
- CRANE R. K., SOLS A. The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J Biol Chem. 1954 Oct;210(2):597–606. [PubMed] [Google Scholar]
- FROMM H. J., ZEWE V. Kinetic studies of the brain hexokinase reaction. J Biol Chem. 1962 May;237:1661–1667. [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V. THE RELATIONSHIPS BETWEEN SUBSTRATES AND ENZYMES OF GLYCOLYSIS IN BRAIN. J Biol Chem. 1964 Jan;239:31–42. [PubMed] [Google Scholar]
- Lowry O. H., Passonneau J. V. Kinetic evidence for multiple binding sites on phosphofructokinase. J Biol Chem. 1966 May 25;241(10):2268–2279. [PubMed] [Google Scholar]
- NEWSHOLME E. A., RANDLE P. J. Regulation of glucose uptake by muscle. 5. Effects of anoxia, insulin, adrenaline and prolonged starving on concentrations of hexose phosphates in isolated rat diaphragm and perfused isolated rat heart. Biochem J. 1961 Sep;80:655–662. doi: 10.1042/bj0800655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Robinson J., Taylor K. A radiochemical enzymatic activity assay for glycerol kinase and hexokinase. Biochim Biophys Acta. 1967 Mar 15;132(2):338–346. doi: 10.1016/0005-2744(67)90153-2. [DOI] [PubMed] [Google Scholar]
- ROSE I. A., O'CONNELL E. L. THE ROLE OF GLUCOSE 6-PHOSPHATE IN THE REGULATION OF GLUCOSE METABOLISM IN HUMAN ERYTHROCYTES. J Biol Chem. 1964 Jan;239:12–17. [PubMed] [Google Scholar]
- Rolleston F. S., Newsholme E. A. Control of glycolysis in cerebral cortex slices. Biochem J. 1967 Aug;104(2):524–533. doi: 10.1042/bj1040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. The inhibition of brain hexokinase by adenosinediphosphate and sulfhydryl reagents. J Biol Chem. 1954 Feb;206(2):925–936. [PubMed] [Google Scholar]
- TIEDEMANN H., BORN J. [Experiments on the mechanism of the Pasteur reaction. The influence of phosphates on the activity of structure-bound hexokinase]. Z Naturforsch B. 1959 Jul;14B:477–478. [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. PROPERTIES OF PHOSPHOFRUCTOKINASE FROM RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLYCOLYSIS AND GLUCONEOGENESIS. Biochem J. 1965 Jun;95:868–875. doi: 10.1042/bj0950868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood A. H., Newsholme E. A. Some properties of phosphofructokinase from kidney cortex and their relation to glucose metabolism. Biochem J. 1967 Jul;104(1):296–299. doi: 10.1042/bj1040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIL-MALHERBE H., BONE A. D. Studies on hexokinase. 1. The hexokinase activity of rat-brain extracts. Biochem J. 1951 Aug;49(3):339–347. doi: 10.1042/bj0490339. [DOI] [PMC free article] [PubMed] [Google Scholar]



