Abstract
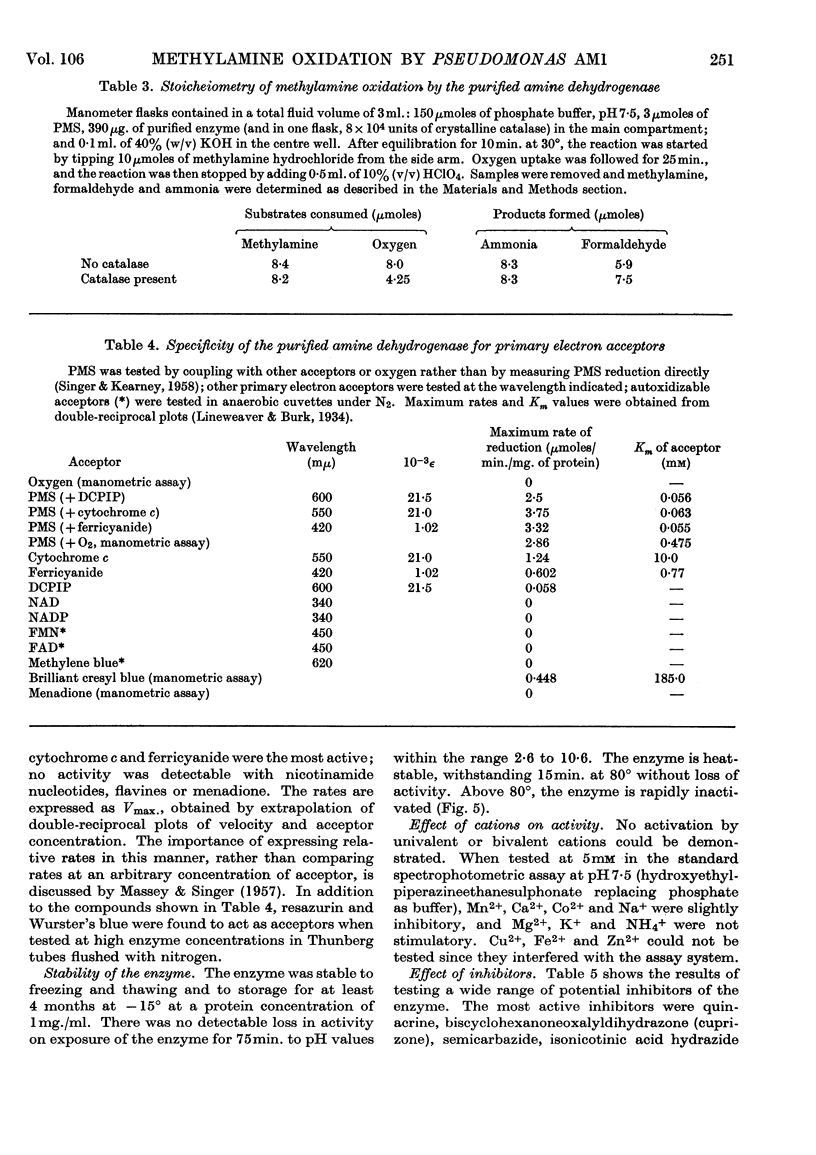
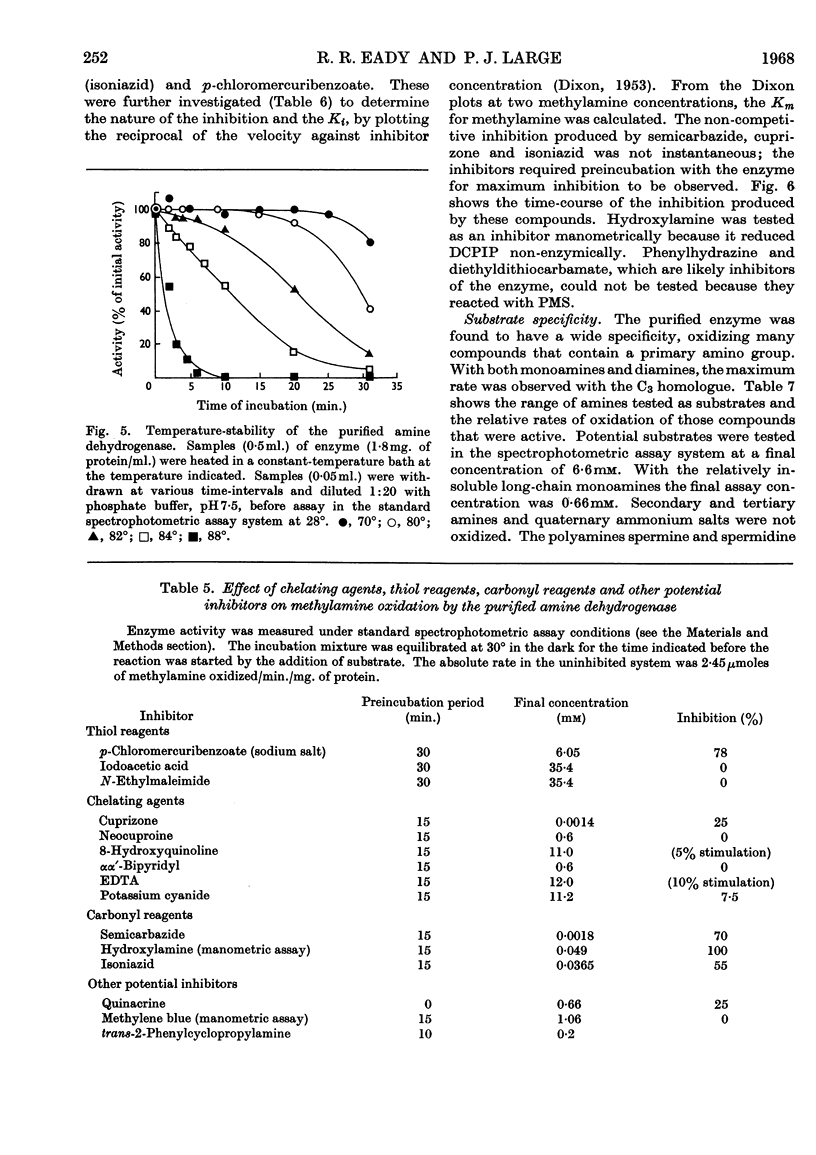
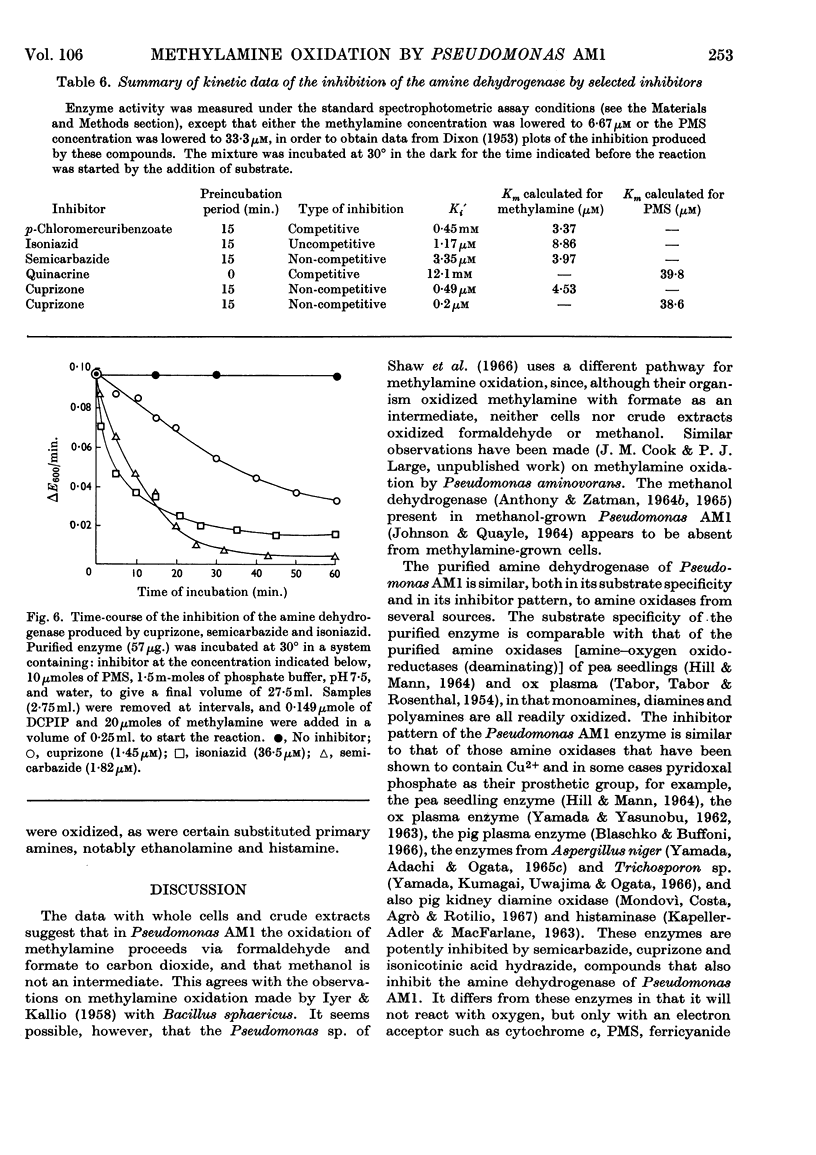
1. Whole cells of Pseudomonas AM1 grown on methylamine oxidize methylamine, formaldehyde and formate. Crude extracts oxidize methylamine only if supplemented with phenazine methosulphate. 2. By using a spectrophotometric assay, the methylamine-oxidizing enzyme has been purified 20-fold in 31% yield. 3. The enzyme is a dehydrogenase, unable to utilize oxygen, NAD, NADP, flavines or menadione as electron acceptors, but able to utilize phenazine methosulphate, ferricyanide, cytochrome c or brilliant cresyl blue. 4. The enzyme is non-specific, readily oxidizing aliphatic monoamines and diamines, histamine and ethanol-amine. Secondary and tertiary amines, quaternary ammonium salts and aromatic amines are not oxidized. 5. The pH optima for methylamine, n-pentylamine and putrescine are respectively 7·6, 8·0 and 8·5. 6. The Km value for methylamine is 5·2μm and that for phenazine methosulphate 56μm. 7. The enzyme will withstand heating for 15min. at 80° without loss of activity, but is inactivated at higher temperatures. It is not inactivated by any pH value between 2·6 and 10·6. 8. The dehydrogenase is inhibited by semicarbazide (Ki 3·35μm), isoniazid (Ki 1·17μm), cuprizone (Ki 0·49μm), p-chloromercuribenzoate (Ki 0·45mm) and quinacrine (Ki 12·1mm). 9. The enzyme is absent from succinate-grown cells, and, during adaptation from succinate to methylamine, activity appears before growth on methylamine begins.
Full text
PDF

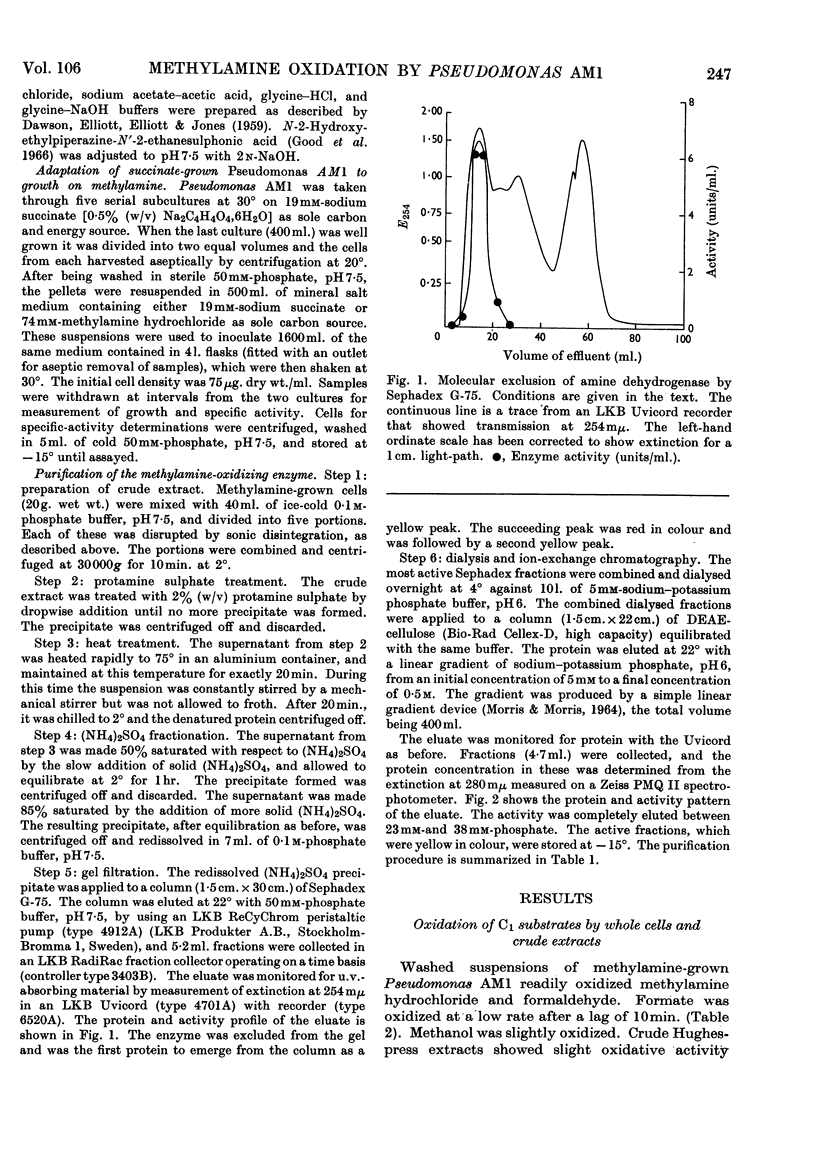
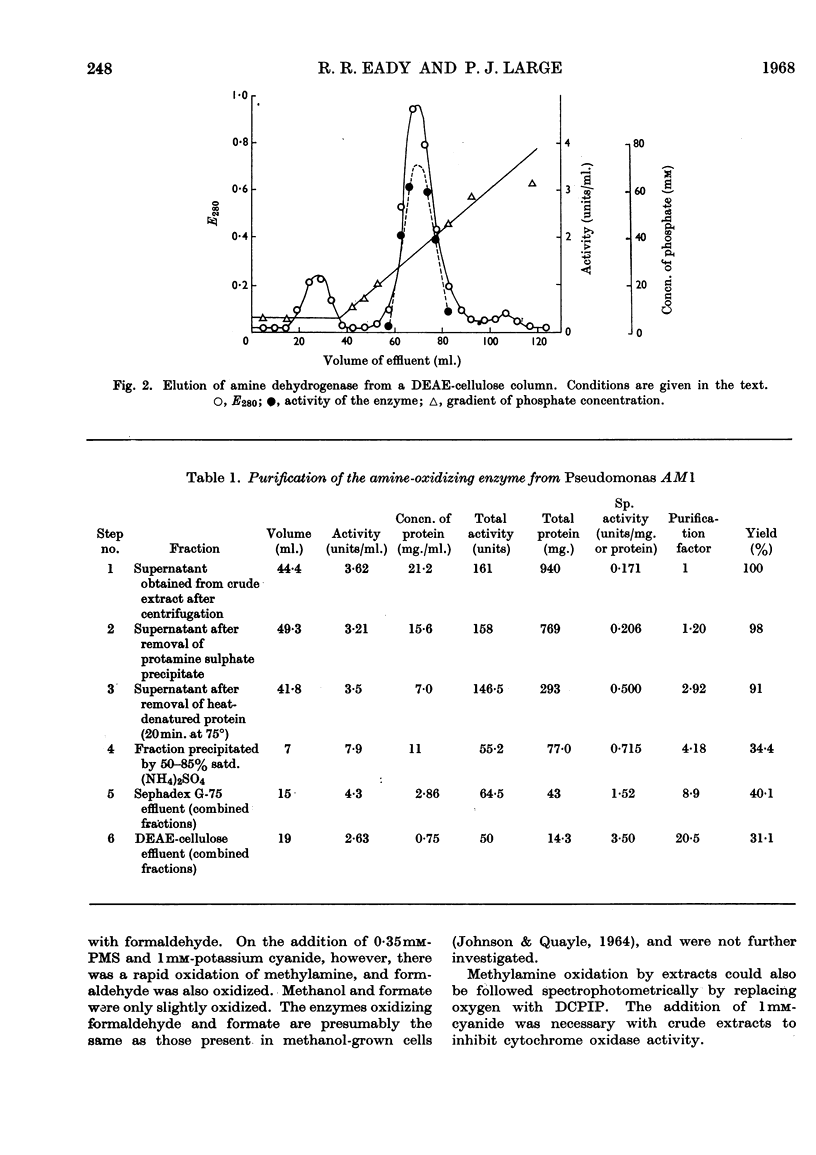
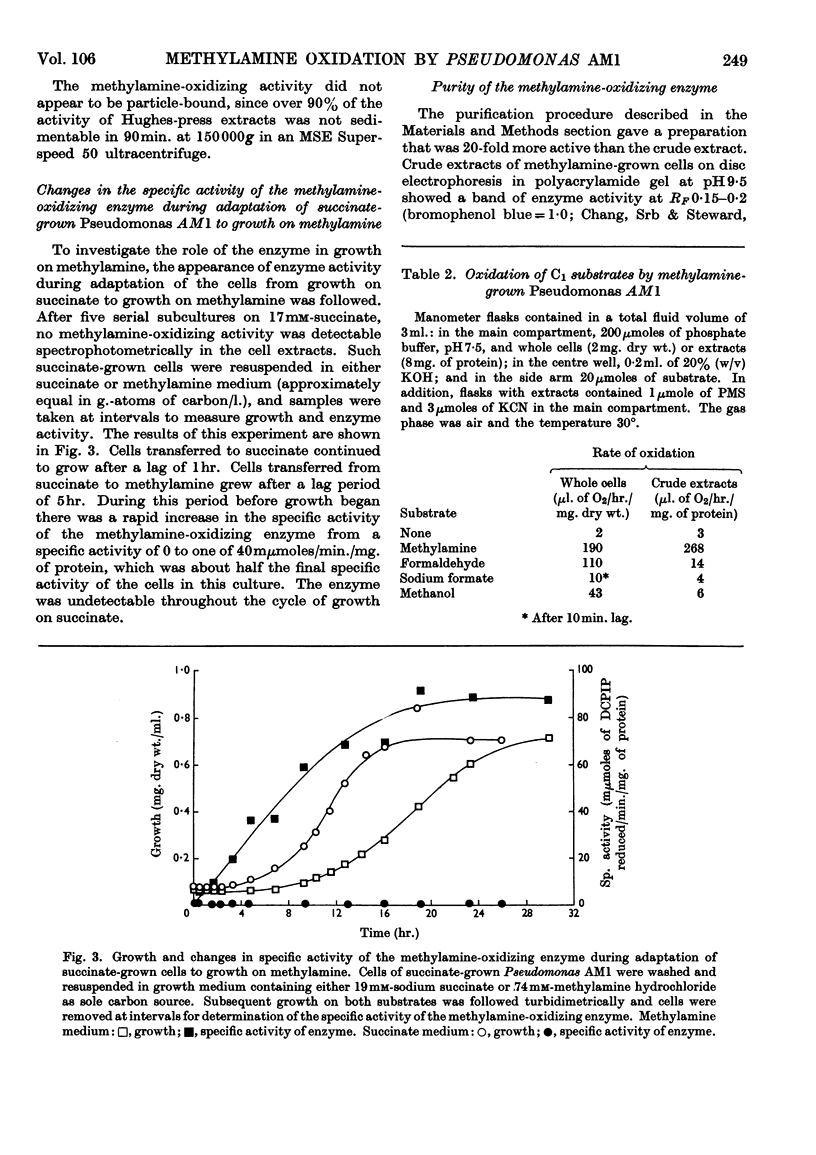
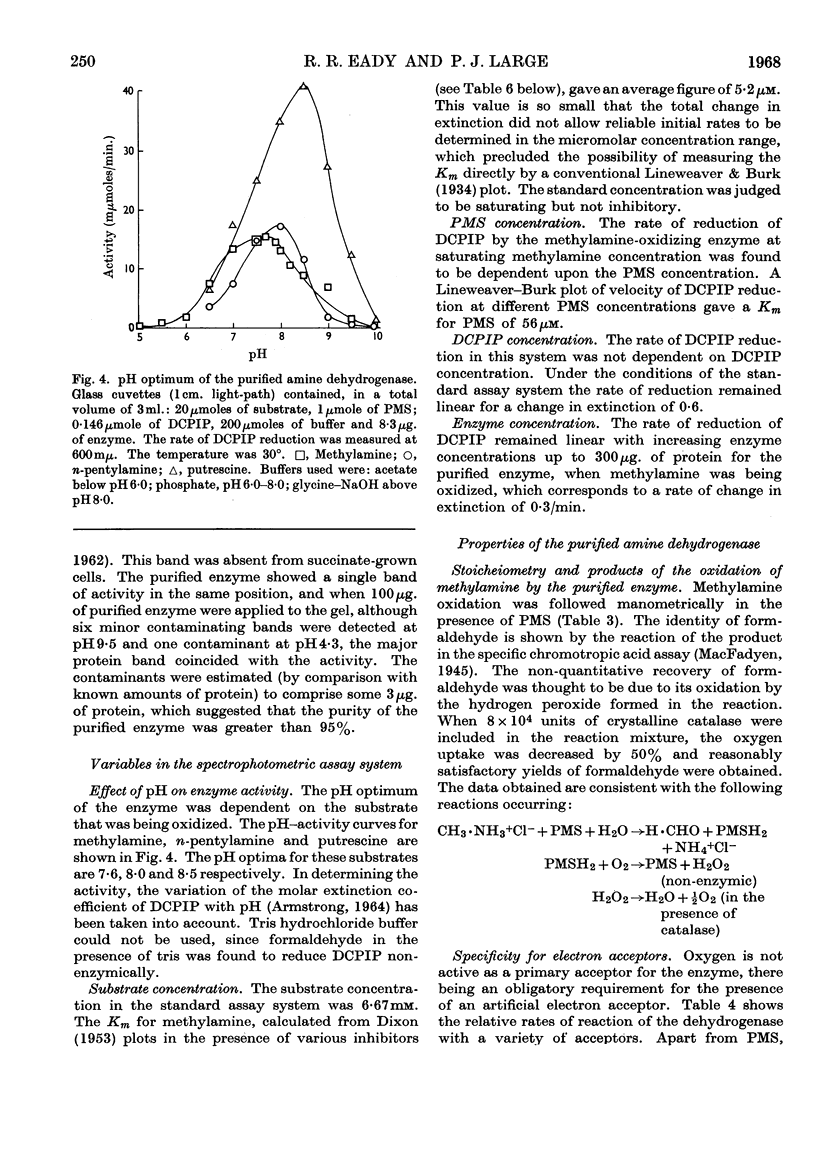


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. M. THE MOLAR EXTINCTION COEFFICIENT OF 2,6-DICHLOROPHENOL INDOPHENOL. Biochim Biophys Acta. 1964 Apr 4;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. 1. Isolation and properties of Pseudomonas sp. M27. Biochem J. 1964 Sep;92(3):609–614. doi: 10.1042/bj0920609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. 2. The methanol-oxidizing enzyme of Pseudomonas sp. M 27. Biochem J. 1964 Sep;92(3):614–621. doi: 10.1042/bj0920614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. The alcohol dehydrogenase of Pseudomonas sp. M27. Biochem J. 1965 Sep;96(3):808–812. doi: 10.1042/bj0960808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACHRACH U. Spermidine oxidase from Serratia marcescens. J Biol Chem. 1962 Nov;237:3443–3448. [PubMed] [Google Scholar]
- BLASCHKO H., BUFFONI F. PYRIDOXAL PHOSPHATE AS A CONSTITUENT OF THE HISTAMINASE (BENZYLAMINE OXIDASE) OF PIG PLASMA. Proc R Soc Lond B Biol Sci. 1965 Aug 24;163:45–60. doi: 10.1098/rspb.1965.0059. [DOI] [PubMed] [Google Scholar]
- BREMNER J. M., KENTEN R. H. Paper chromatography of amines. Biochem J. 1951 Oct;49(5):651–655. doi: 10.1042/bj0490651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- CHANG L. O., SRB A. M., STEWARD F. C. Electrophoretic separations of the soluble proteins of Neurospora. Nature. 1962 Feb 24;193:756–759. doi: 10.1038/193756a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBIN D. T. The assay and characterization of amines by 2,4-dinitrofluorobenzene. J Biol Chem. 1960 Mar;235:783–786. [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- Hill J. M., Mann P. J. Further properties of the diamine oxidase of pea seedlings. Biochem J. 1964 Apr;91(1):171–182. doi: 10.1042/bj0910171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER S. N., KALLIO R. E. Bacterial degradation of methylurea. Arch Biochem Biophys. 1958 Aug;76(2):295–305. doi: 10.1016/0003-9861(58)90155-3. [DOI] [PubMed] [Google Scholar]
- JAYASURIYA G. C. The isolation and characteristics of an oxalate-decomposing organism. J Gen Microbiol. 1955 Jun;12(3):419–428. doi: 10.1099/00221287-12-3-419. [DOI] [PubMed] [Google Scholar]
- Johnson P. A., Quayle J. R. Microbial growth on C-1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol-grown Pseudomonas AM-1. Biochem J. 1964 Nov;93(2):281–290. doi: 10.1042/bj0930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARGE P. J., PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. II. Synthesis of cell constituents by methanol- and formate-grown Pseudomonas AM 1, and methanol-grown Hyphomicrobium vulgare. Biochem J. 1961 Dec;81:470–480. doi: 10.1042/bj0810470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASSEY V., SINGER T. P. Studies on succinic dehydrogenase. VI. The reactivity of beef heart succinic dehydrogenase with electron carriers. J Biol Chem. 1957 Dec;229(2):755–762. [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- Mondovì B., Costa M. T., Agrò A. F., Rotilio G. Pyridoxal phosphate as a prosthetic group of pig kidney diamine oxidase. Arch Biochem Biophys. 1967 Mar;119(1):373–381. doi: 10.1016/0003-9861(67)90468-7. [DOI] [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara S., Gomes B., Yasunobu K. T. Amine oxidase. VII. Beef liver mitochondrial monoamine oxidase, a copper-containing protein. J Biol Chem. 1966 Jun 25;241(12):2774–2780. [PubMed] [Google Scholar]
- PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. I. Isolation and characterization of Pseudomonas AM 1. Biochem J. 1961 Dec;81:465–469. doi: 10.1042/bj0810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B. Determination of succinic dehydrogenase activity. Methods Biochem Anal. 1957;4:307–333. doi: 10.1002/9780470110201.ch9. [DOI] [PubMed] [Google Scholar]
- Shaw W. V., Tsai L., Stadtman E. R. The enzymatic synthesis of N-methylglutamic acid. J Biol Chem. 1966 Feb 25;241(4):935–945. [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- YAMADA H., YASUNOBU K. T. MONOAMINE OXIDASE. IV. NATURE OF THE SECOND PROSTHETIC GROUP OF PLASMA MONOAMINE OXIDASE. J Biol Chem. 1963 Aug;238:2669–2675. [PubMed] [Google Scholar]
- YAMADA H., YASUNOBU K. T. Monoamine oxidase. II. Copper, one of the prosthetic groups of plasma monoamine oxidase. J Biol Chem. 1962 Oct;237:3077–3082. [PubMed] [Google Scholar]


