Abstract
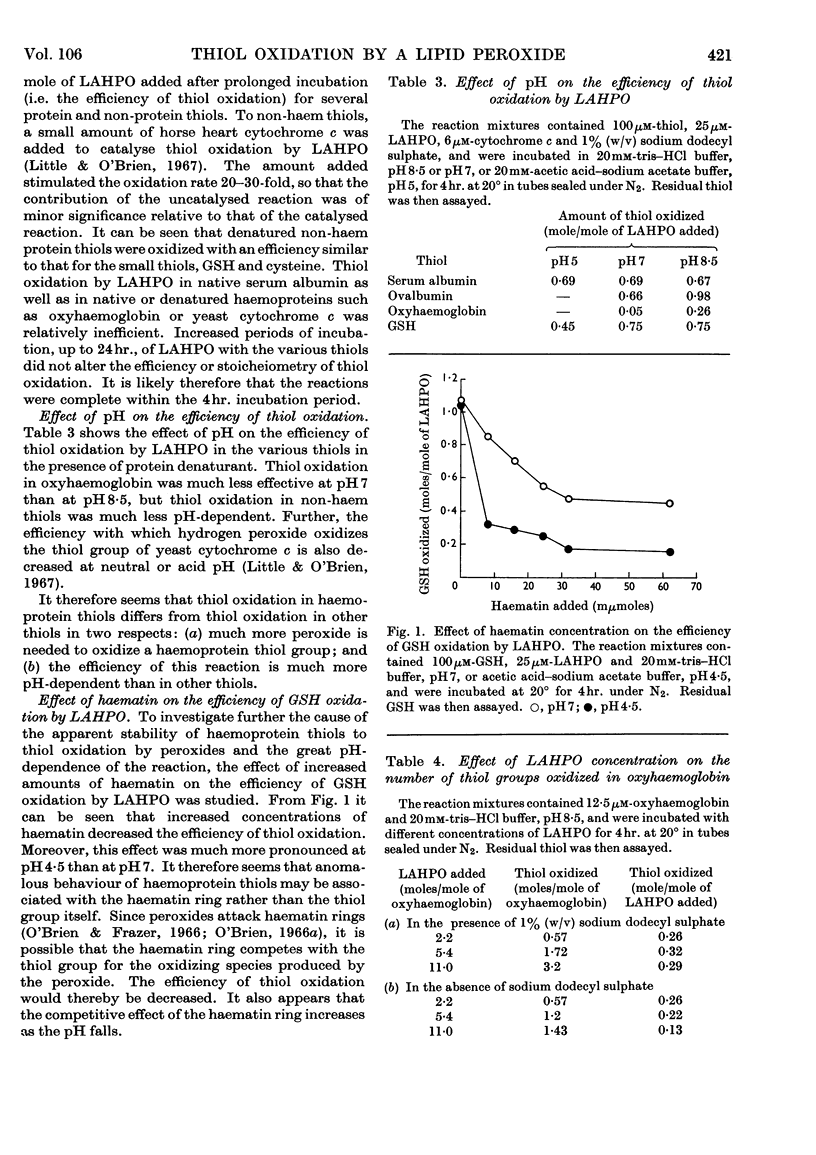
1. Thiol oxidation by a lipid peroxide or hydrogen peroxide was as efficient in denatured non-haem proteins as in small thiols. Both peroxides were relatively ineffective in oxidizing haemoprotein thiols, especially at low pH. Increased amounts of haematin decreased greatly the efficiency of GSH oxidation by peroxides especially at low pH. 2. Other than the haematin ring, the thiol group was found to be probably the group in proteins most sensitive to modification by peroxides. 3. At low concentrations, the fatty acid moiety of a lipid peroxide appeared to impede thiol oxidation in proteins, probably by hydrophobic bonding to the protein, rather than to stimulate thiol oxidation by denaturing the protein and thereby increasing the exposure and reactivity of the thiol group. 4. The relative rates of thiol oxidation by peroxides in the different thiols were: haemoprotein thiols>small thiols>other protein thiols. In all cases, thiol oxidation was much more rapid by the lipid peroxide than by hydrogen peroxide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., CECIL R. The thiol groups of normal adult human haemoglobin. Biochem J. 1958 May;69(1):27–34. doi: 10.1042/bj0690027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiancone E., Gilbert G. A. Dissociation of hemoglobin into subunits. I. Oxyhemoglobin: effect of acetic acid. J Biol Chem. 1965 Oct;240(10):3866–3867. [PubMed] [Google Scholar]
- HAGIHARA B., HORIO T., NOZAKI M., OKUNUKI K., YAMASHITA J. Crystalline cytochrome c: preparation of crystalline cytochrome c from yeast. Nature. 1956 Sep 22;178(4534):629–630. doi: 10.1038/178629b0. [DOI] [PubMed] [Google Scholar]
- McKnight R. C., Hunter F. E., Jr Mitochondrial membrane ghosts produced by lipid peroxidation induced by ferrous ion. II. Composition and enzymatic activity. J Biol Chem. 1966 Jun 25;241(12):2757–2765. [PubMed] [Google Scholar]
- O'Brien P. J., Frazer A. C. The effect of lipid peroxides on the biochemical constituents of the cell. Proc Nutr Soc. 1966;25(1):9–18. doi: 10.1079/pns19660005. [DOI] [PubMed] [Google Scholar]
- OTTOLENGHI A., BERNHEIM F., WILBUR K. M. The inhibition of certain mitochondrial enzymes by fatty acids oxidized by ultraviolet light or ascorbic acid. Arch Biochem Biophys. 1955 May;56(1):157–164. doi: 10.1016/0003-9861(55)90345-3. [DOI] [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E. Purification and cystallization of the myoglobin of salt water fish. Arch Biochem Biophys. 1955 Oct;58(2):498–500. doi: 10.1016/0003-9861(55)90148-x. [DOI] [PubMed] [Google Scholar]
- Recknagel R. O., Ghoshal A. K. Lipoperoxidation of rat liver microsomal lipids induced by carbon tetrachloride. Nature. 1966 Jun 11;210(5041):1162–1163. doi: 10.1038/2101162a0. [DOI] [PubMed] [Google Scholar]
- Roubal W. T., Tappel A. L. Damage to proteins, enzymes, and amino acids by peroxidizing lipids. Arch Biochem Biophys. 1966 Jan;113(1):5–8. doi: 10.1016/0003-9861(66)90150-0. [DOI] [PubMed] [Google Scholar]


