Abstract
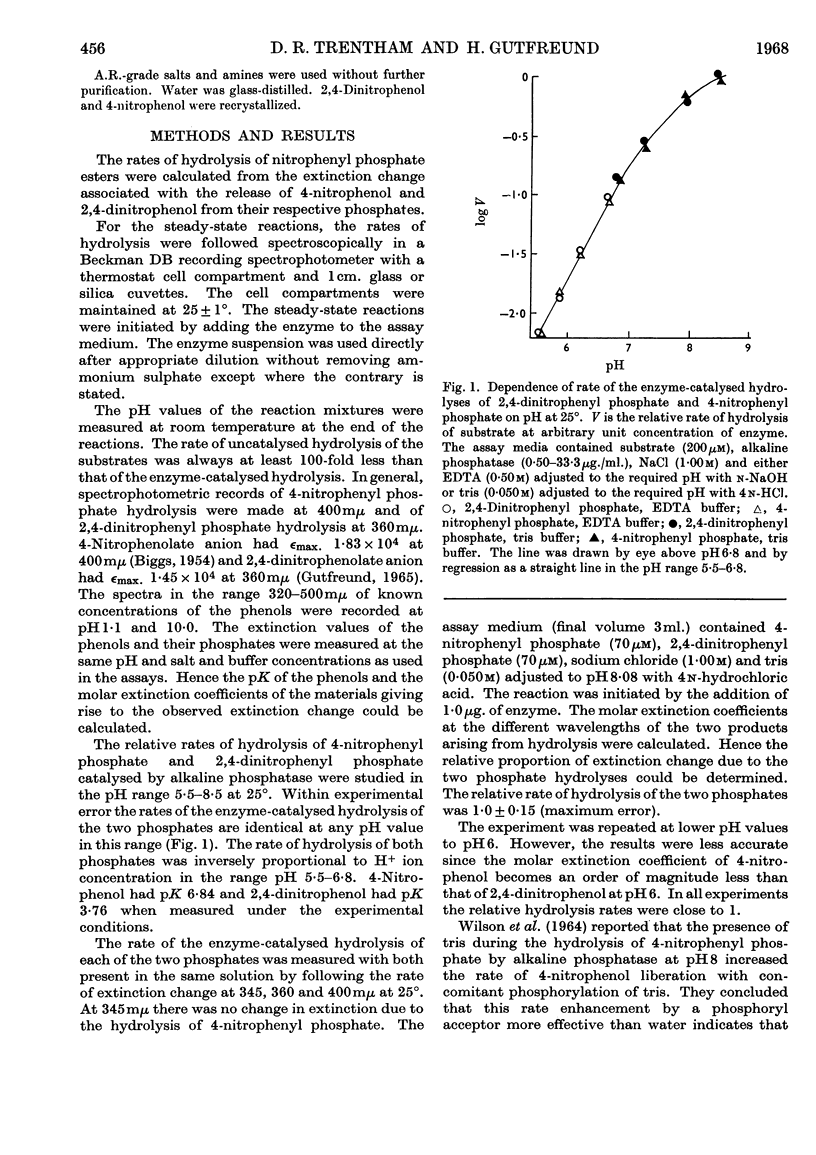
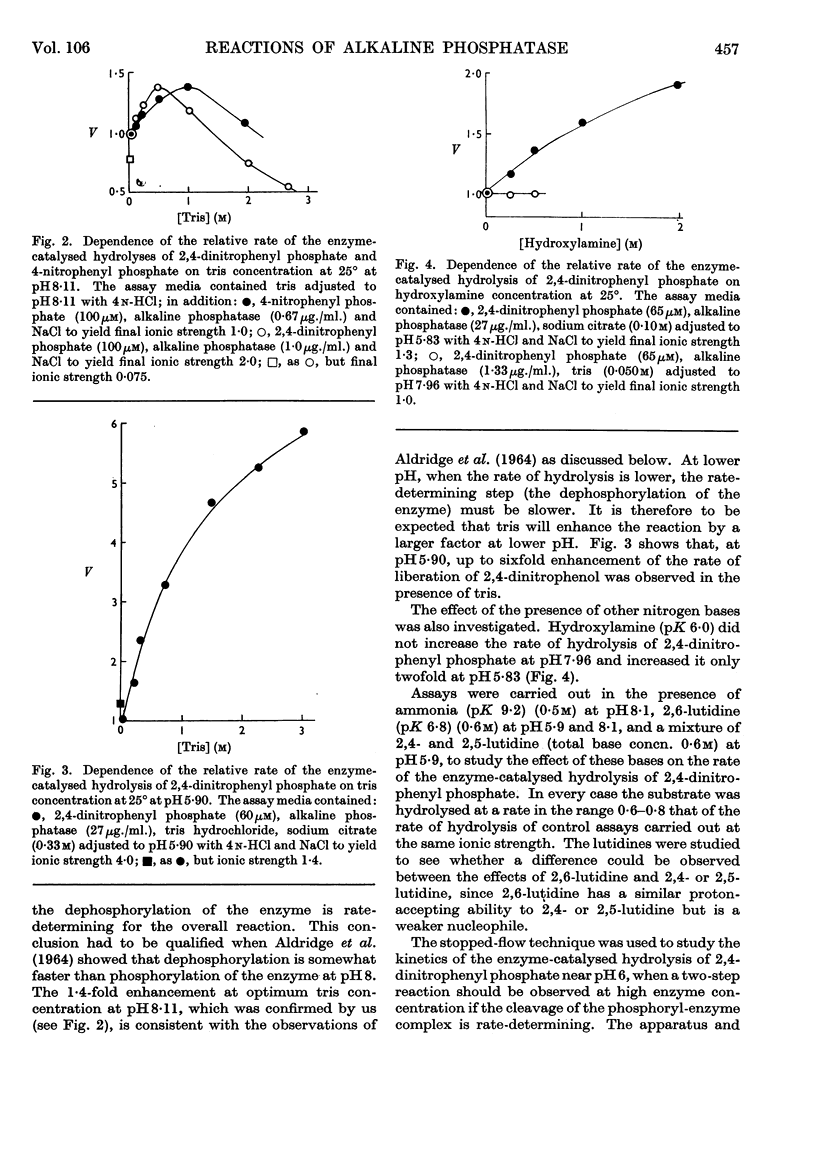
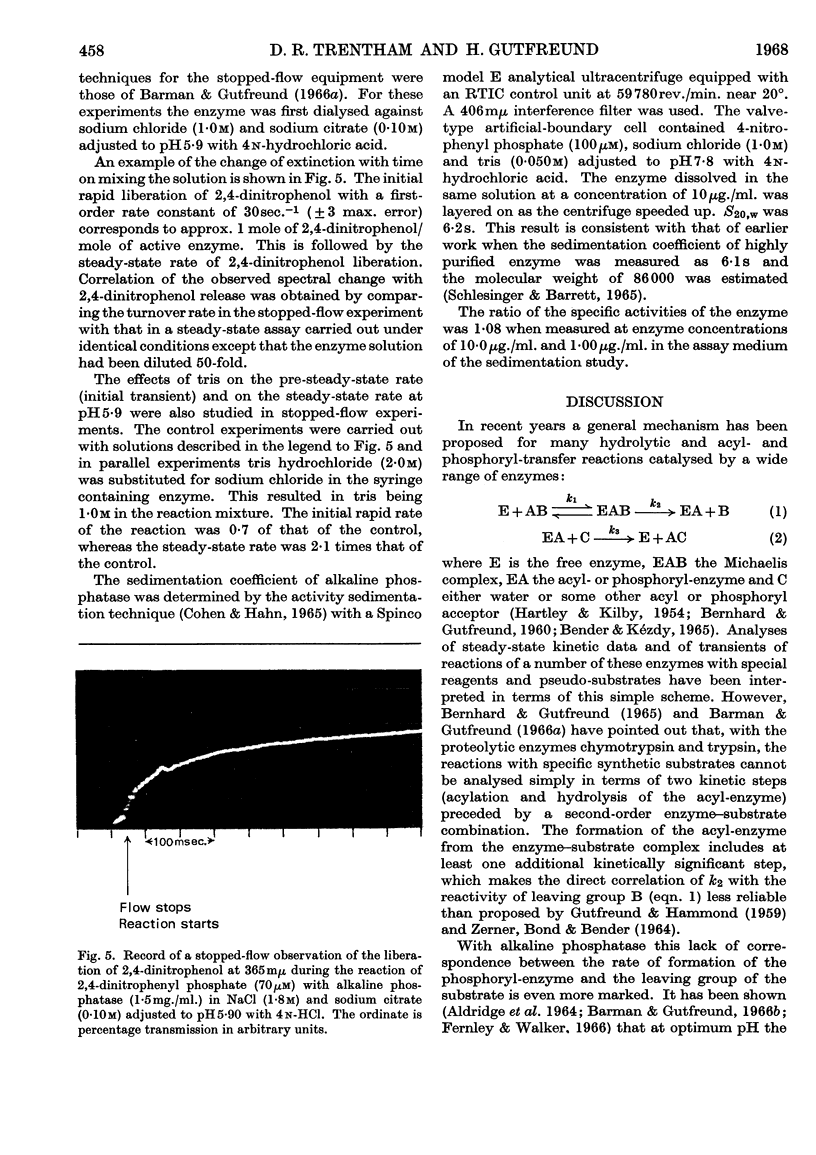
1. The steady-state rate of hydrolysis of 2,4-dinitrophenyl phosphate catalysed by Escherichia coli phosphatase is identical with that of 4-nitrophenyl phosphate over the pH range 5·5–8·5. 2. The increase in the rate of the enzyme-catalysed decomposition of nitrophenyl phosphates in the presence of tris at pH8·1 and 5·9 is consistent with the hypothesis that tris increases the rate of decomposition of a phosphoryl-enzyme intermediate. At pH8·1 the rate of decomposition of the phosphoryl-enzyme is approximately twice as fast as the rate of its formation, whereas at pH5·9 the rate of formation of the phosphoryl-enzyme is considerably faster than its decomposition. 3. Pre-steady-state measurements of the initial transient of the liberation of 2,4-dinitrophenol during the reaction of the enzyme with 2,4-dinitrophenyl phosphate confirmed the above pH-dependence of the ratio of the rates of phosphorylation and dephosphorylation of the enzyme. At optimum pH (above pH8), when the phosphorylation of the enzyme by the substrate is rate-determining, this step must be controlled by a rearrangement of the enzyme or enzyme–substrate complex.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Nordlie R. C. Inorganic pyrophosphate-glucose phosphotransferase activity associated with alkaline phosphatase of Escherichia coli. J Biol Chem. 1967 Jan 10;242(1):114–119. [PubMed] [Google Scholar]
- Barman T. E., Gutfreund H. Optical and chemical identification of kinetic steps in trypsin- and chymotrypsin-catalysed reactions. Biochem J. 1966 Nov;101(2):411–416. doi: 10.1042/bj1010411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman T. E., Gutfreund H. The catalytic-centre activity and kinetic properties of bovine milk alkaline phosphatase. Biochem J. 1966 Nov;101(2):460–466. doi: 10.1042/bj1010460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard S. A., Gutfreund H. The optical detection of transients in trypsin- and chymotrypsin-catalyzed reactions. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1238–1243. doi: 10.1073/pnas.53.6.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTFREUND H., HAMMOND B. R. Steps in the reactions of chymotrypsin with tyrosine derivatives. Biochem J. 1959 Nov;73:526–530. doi: 10.1042/bj0730526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTLEY B. S., KILBY B. A. The reaction of p-nitrophenyl esters with chymotrypsin and insulin. Biochem J. 1954 Feb;56(2):288–297. doi: 10.1042/bj0560288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- PIGRETTI M. M., MILSTEIN C. ACID INACTIVATION OF AND INCORPORATION OF PHOSPHATE INTO ALKALINE PHOSPHATASE FROM ESCHERICHIA COLI. Biochem J. 1965 Jan;94:106–113. doi: 10.1042/bj0940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Barrett K. The reversible dissociation of the alkaline phosphatase of Escherichia coli. I. Formation and reactivation of subunits. J Biol Chem. 1965 Nov;240(11):4284–4292. [PubMed] [Google Scholar]
- WILSON I. B., DAYAN J., CYR K. SOME PROPERTIES OF ALKALINE PHOSPHATASE FROM ESCHERICHIA COLI. TRANSPHOSPHORYLATION. J Biol Chem. 1964 Dec;239:4182–4185. [PubMed] [Google Scholar]


