Abstract
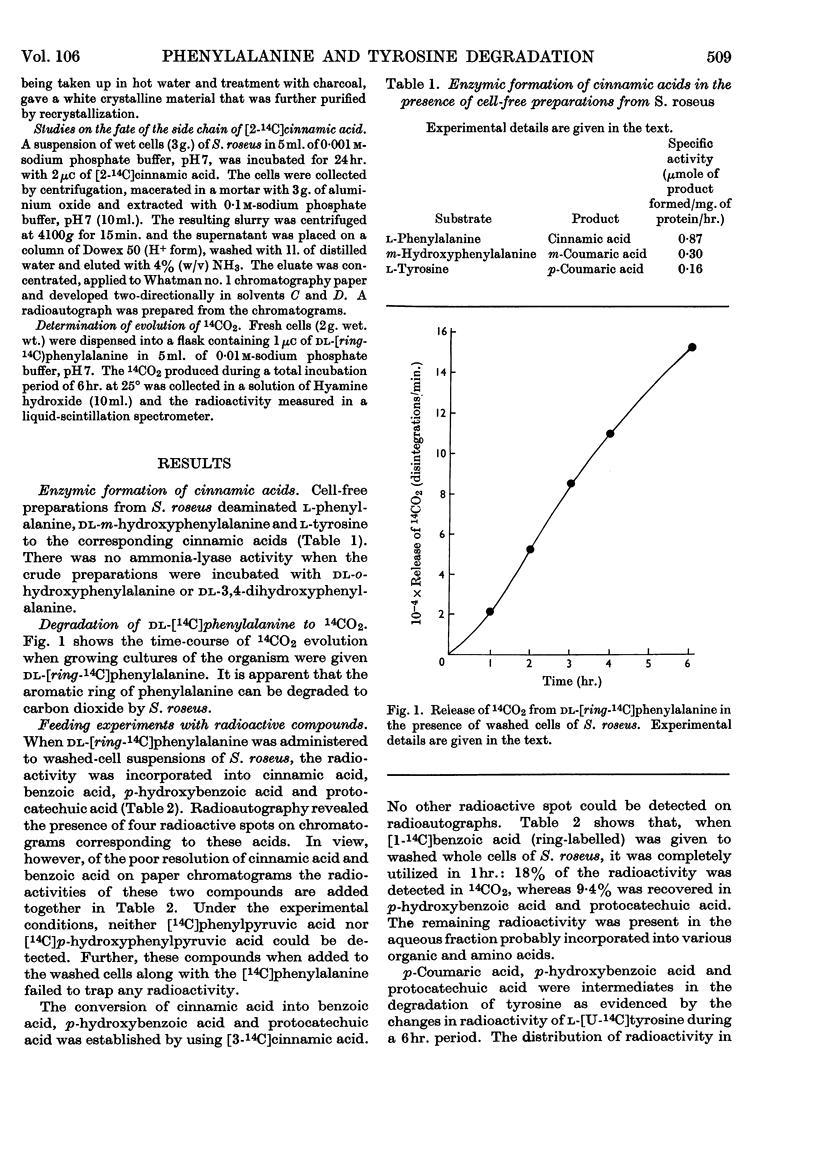
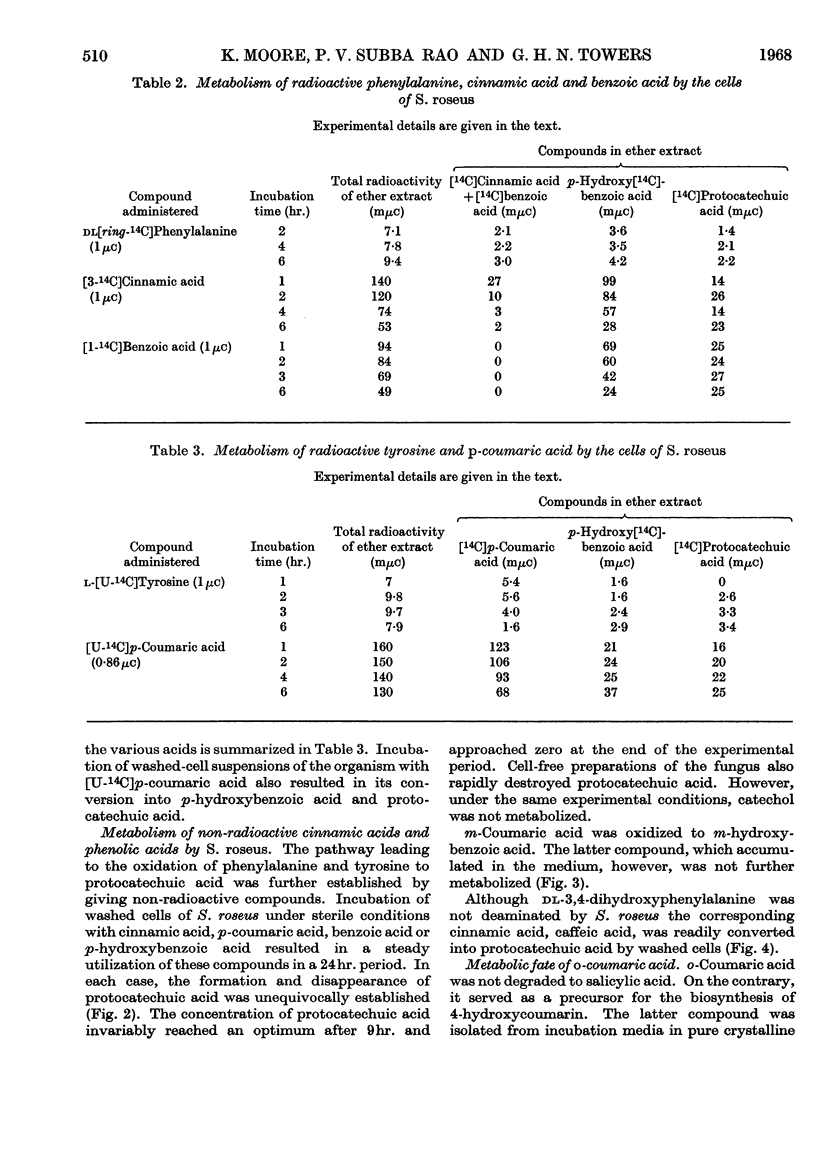
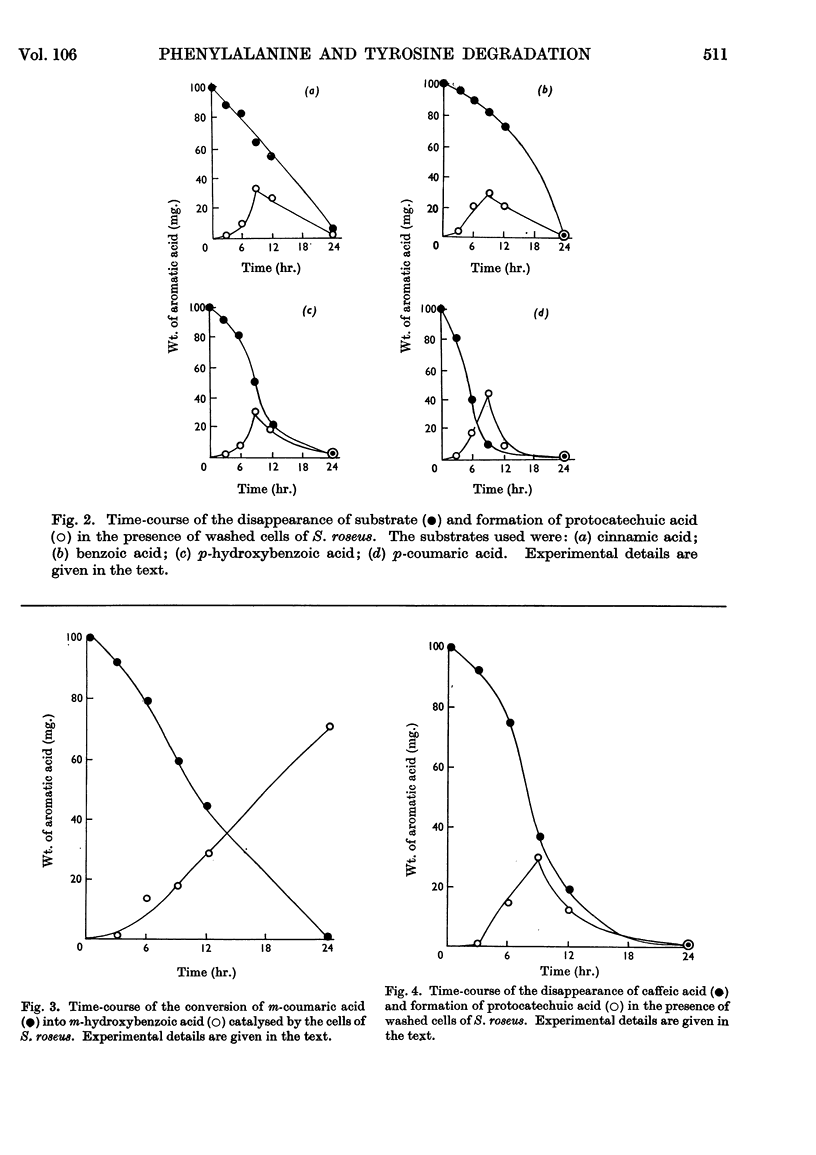
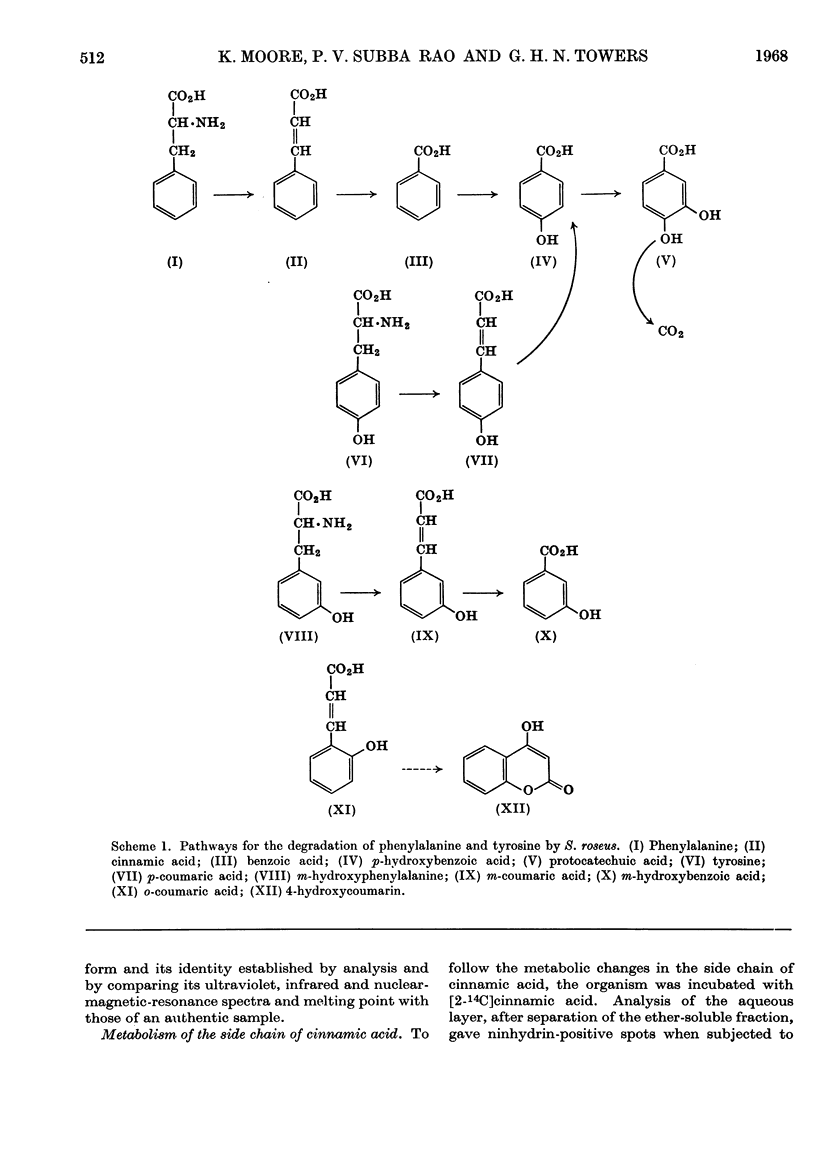
Ammonia-lyase activity for l-phenylalanine, m-hydroxyphenylalanine and l-tyrosine was demonstrated in cell-free extracts of Sporobolomyces roseus. Cultures of this organism converted dl-[ring-14C]phenylalanine and l-[U-14C]tyrosine into the corresponding cinnamic acid. Tracer studies showed that these compounds were further metabolized to [14C]protocatechuic acid. Benzoic acid and p-hydroxybenzoic acid were intermediates in this pathway. Washed cells of the organism readily utilized cinnamic acid, p-coumaric acid, caffeic acid, benzoic acid and p-hydroxybenzoic acid. Protocatechuic acid was the terminal aromatic compound formed during the metabolism of these compounds. The cells of S. roseus were able to convert m-coumaric acid into m-hydroxybenzoic acid, but the latter compound, which accumulated in the medium, was not further metabolized. 4-Hydroxycoumarin was identified as the product of o-coumaric acid metabolism by this organism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLAKLEY E. R., SIMPSON F. J. THE MICROBIAL METABOLISM OF CINNAMIC ACID. Can J Microbiol. 1964 Apr;10:175–185. doi: 10.1139/m64-025. [DOI] [PubMed] [Google Scholar]
- Dagley S., Chapman P. J., Gibson D. T. The metabolism of beta-phenylpropionic acid by an Achromobacter. Biochem J. 1965 Dec;97(3):643–650. doi: 10.1042/bj0970643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL-BAGOURY S., FLETCHER S., MORRISON R. B. Effect of chloramphenicol in maintaining the viability of Escherichia coli. Nature. 1956 Dec 29;178(4548):1467–1467. doi: 10.1038/1781467a0. [DOI] [PubMed] [Google Scholar]
- EVANS W. C. THE MICROBIOLOGICAL DEGRADATION OF AROMATIC COMPOUNDS. J Gen Microbiol. 1963 Aug;32:177–184. doi: 10.1099/00221287-32-2-177. [DOI] [PubMed] [Google Scholar]
- FINKLE B. J., LEWIS J. C., CORSE J. W., LUNDIN R. E. Enzyme reactions with phenolic compounds: formation of hydroxystyrenes through the decarboxylation of 4-hydroxycinnamic acids by Aerobacter. J Biol Chem. 1962 Sep;237:2926–2931. [PubMed] [Google Scholar]
- HENDERSON M. E., FARMER V. C. Utilization by soil fungi of p-hydroxybenzaidehyde, ferulic acid, syringaldehyde and vanillin. J Gen Microbiol. 1955 Feb;12(1):37–46. doi: 10.1099/00221287-12-1-37. [DOI] [PubMed] [Google Scholar]
- HENDERSON M. E. The metabolism of aromatic compounds related to lignin by some hyphomycetes and yeast-like fungi of soil. J Gen Microbiol. 1961 Sep;26:155–165. doi: 10.1099/00221287-26-1-155. [DOI] [PubMed] [Google Scholar]
- IBRAHIM R. K., TOWERS G. H. The identification, by chromatography, of plant phenolic acids. Arch Biochem Biophys. 1960 Mar;87:125–128. doi: 10.1016/0003-9861(60)90132-6. [DOI] [PubMed] [Google Scholar]
- KLUYVER A. J., VAN ZIJP J. C. The production of homogentisic acid out of phenylacetic acid by Aspergillus niger. Antonie Van Leeuwenhoek. 1951;17(5):315–324. doi: 10.1007/BF02062278. [DOI] [PubMed] [Google Scholar]
- KOUKOL J., CONN E. E. The metabolism of aromatic compounds in higher plants. IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare. J Biol Chem. 1961 Oct;236:2692–2698. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MEAD J. A., SMITH J. N., WILLIAMS R. T. Studies in detoxication. 72. The metabolism of coumarin and of o-coumaric acid. Biochem J. 1958 Jan;68(1):67–74. doi: 10.1042/bj0680067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAIR P. M., VAIDYANATHAN C. S. A COLORIMETRIC METHOD FOR DETERMINATION OF PYROCATECHOL AND RELATED SUBSTANCES. Anal Biochem. 1964 Mar;7:315–321. [PubMed] [Google Scholar]
- PITTARD A. J., GIBSON F., DOY C. H. A possible relationship between the formation of o-dihydric phenols and tryptophan biosynthesis by Aerobacter aerogens. Biochim Biophys Acta. 1962 Feb 26;57:290–298. doi: 10.1016/0006-3002(62)91122-8. [DOI] [PubMed] [Google Scholar]
- Power D. M., Towers G. H., Neish A. C. Biosynthesis of phenolic acids by certain wood-destroying basidiomycetes. Can J Biochem. 1965 Sep;43(9):1397–1407. doi: 10.1139/o65-157. [DOI] [PubMed] [Google Scholar]
- ROGOFF M. H. Oxidation of aromatic compounds by bacteria. Adv Appl Microbiol. 1961;3:193–221. doi: 10.1016/s0065-2164(08)70510-0. [DOI] [PubMed] [Google Scholar]
- Vollmer K. O., Reisener H. J., Grisebach H. The formation of acetic acid from p-hydroxycinnamic acid during its degradation to p-hydroxybenzoic acid in wheat shoots. Biochem Biophys Res Commun. 1965 Nov 8;21(3):221–225. doi: 10.1016/0006-291x(65)90275-5. [DOI] [PubMed] [Google Scholar]
- WEBLEY D. M., DUFF R. B., FARMER V. C. Beta-oxidation of fatty acids by Nocardia opaca. J Gen Microbiol. 1955 Oct;13(2):361–369. doi: 10.1099/00221287-13-2-361. [DOI] [PubMed] [Google Scholar]
- WEBLEY D. M., DUFF R. B., FARMER V. C. The influence of chemical structure on beta-oxidation by soil nocardias. J Gen Microbiol. 1958 Jun;18(3):733–746. doi: 10.1099/00221287-18-3-733. [DOI] [PubMed] [Google Scholar]
- WHITING G. C., CARR J. G. Metabolism of cinnamic acid and hydroxy-cinnamic acids by Lactobacillus pastorianus var. quinicus. Nature. 1959 Oct 31;184(Suppl 18):1427–1428. doi: 10.1038/1841427a0. [DOI] [PubMed] [Google Scholar]


