Abstract
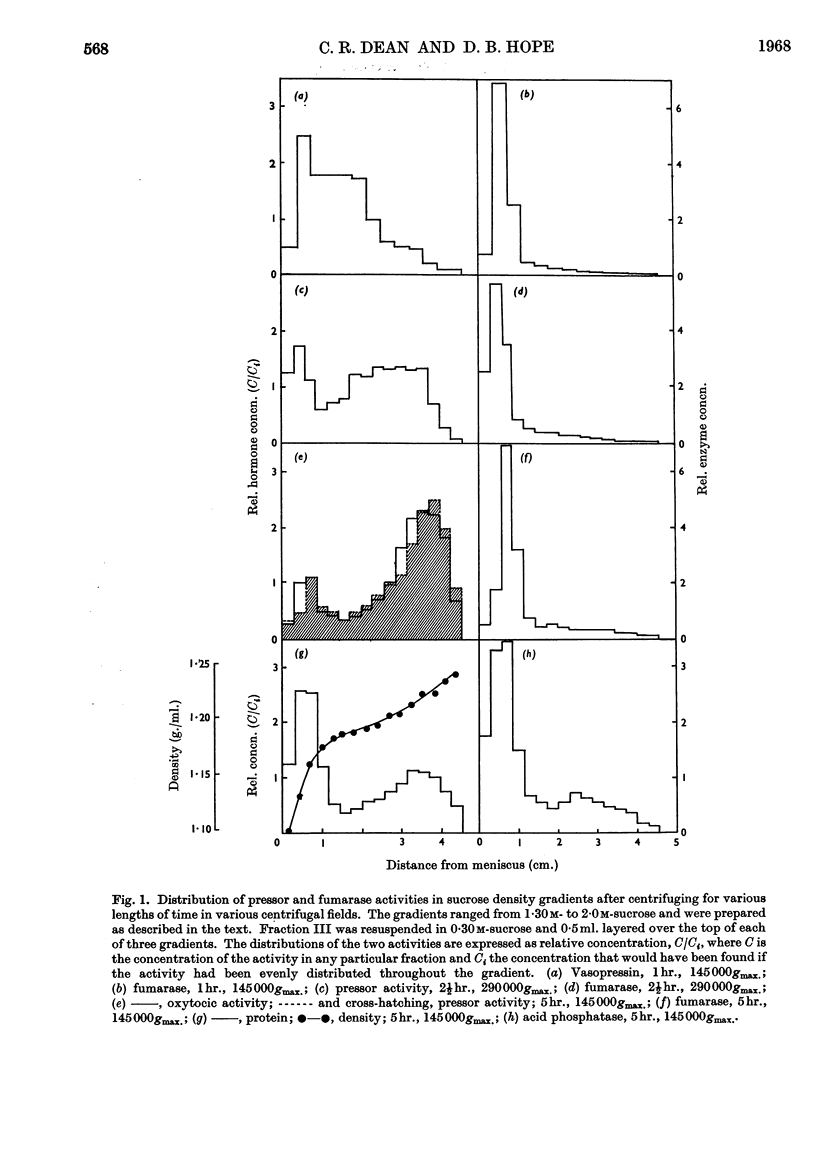
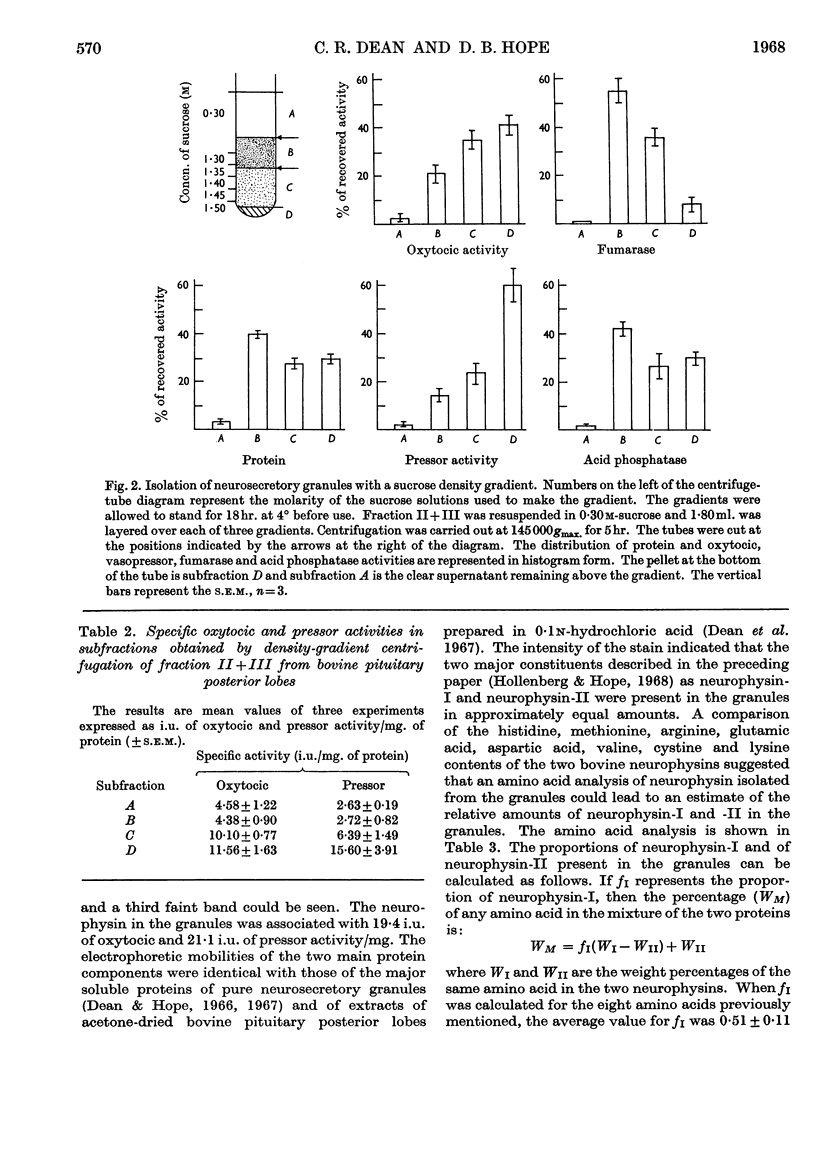
1. An improved procedure for the isolation of neurosecretory granules from the posterior lobe of the bovine pituitary gland is described. 2. Of the total oxytocic and pressor activities present in the original tissue 80% was sedimentable. 3. The granules were separated from mitochondria by prolonged centrifugation in a sucrose density gradient. During a sedimentation period of 5hr. the granules moved progressively into denser regions of the gradient and the mitochondria remained at the top. 4. The biological activities of the granules were measured: the oxytocic activity was 11·56±1·63 and the pressor activity was 15·60±3·91 units/mg. of protein. 5. A protein was isolated from a lysate of granules prepared from 40 pituitary glands. Amino acid analysis showed that it consisted of a mixture of neurophysin-I and neurophysin-II in equal proportions. It accounted for 60% of the soluble granule protein and for 50% of the total granule protein. 6. The neurophysins present in the granules are associated with 19·1 units of oxytocic and 21·1 units of pressor activity/mg. of protein. 7. Starch-gel electrophoresis revealed the presence of both neurophysins in extracts of 15 pituitary glands studied individually. 8. We conclude that the polypeptide hormones, oxytocin and [8-arginine]-vasopressin, are normally closely associated with the two neurophysins within neurosecretory granules of the pituitary gland.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANGHAM D. R., MUSSETT M. V. Third international standard for posterior pituitary; re-named third international standard for oxytocic, vasopressor and antidiuretic substances in 1956. Bull World Health Organ. 1958;19(2):325–340. [PMC free article] [PubMed] [Google Scholar]
- BARER R., HELLER H., LEDERIS K. THE ISOLATION, IDENTIFICATION AND PROPERTIES OF THE HORMONAL GRANULES OF THE NEUROHYPOPHYSIS. Proc R Soc Lond B Biol Sci. 1963 Oct 22;158:388–416. doi: 10.1098/rspb.1963.0054. [DOI] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., De Duve C. Combined biochemical and morphological study of particulate fractions from rat liver. Analysis of preparations enriched in lysosomes or in particles containing urate oxidase, D-amino acid oxidase, and catalase. J Cell Biol. 1965 Jul;26(1):219–243. doi: 10.1083/jcb.26.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindler E., Labella F. S., Sanwal M. Isolated nerve endings (neurosecretosomes) from the posterior pituitary. Partial separation of vasopressin and oxytocin and the isolation of microvesicles. J Cell Biol. 1967 Jul;34(1):185–205. doi: 10.1083/jcb.34.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- DE ROBERTIS E., PELLEGRINO DE IRALDI A., RODRIGUEZ G., GOMEZ C. J. On the isolation of nerve endings and synaptic vesicles. J Biophys Biochem Cytol. 1961 Jan;9:229–235. doi: 10.1083/jcb.9.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEKANSKI J. The quantitative assay of vasopressin. Br J Pharmacol Chemother. 1952 Dec;7(4):567–572. doi: 10.1111/j.1476-5381.1952.tb00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. R., Hope D. B. The isolation of purified neurosecretory granules from bovine pituitary posterior lobes. Comparison of granule protein constituents with those of neurophysin. Biochem J. 1967 Sep;104(3):1082–1088. doi: 10.1042/bj1041082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON K. A., WALLACE A. L. Starch-gel electrophoresis of anterior pituitary hormones. Nature. 1961 May 13;190:629–630. doi: 10.1038/190629a0. [DOI] [PubMed] [Google Scholar]
- GAITAN E., COBO E., MIZRACHI M. EVIDENCE FOR THE DIFFERENTIAL SECRETION OF OXYTOCIN AND VASOPRESSIN IN MAN. J Clin Invest. 1964 Dec;43:2310–2322. doi: 10.1172/JCI105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Hope D. B. Fractionation of neurophysin by molecular-sieve and ion-exchange chromatography. Biochem J. 1967 Jul;104(1):122–127. doi: 10.1042/bj1040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Hope D. B. The isolation of the native hormone-binding proteins from bovine pituitary posterior lobes. Crystallization of neurophysin-I and-II as complexes with [8-arginine]-vasopressin. Biochem J. 1968 Jan;106(2):557–564. doi: 10.1042/bj1060557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABELLA F. S., REIFFENSTEIN R. J., BEAULIEU G. Subcellular fractionation of bovine posterior pituitary glands by centrifugation. Arch Biochem Biophys. 1963 Mar;100:399–408. doi: 10.1016/0003-9861(63)90104-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaBella F. S., Sanwal M. Isolation of nerve endings from the posterior pituitary gland. Electron microscopy of fractions obtained by centrifugation. J Cell Biol. 1965 Jun;25(3 Suppl):179–193. doi: 10.1083/jcb.25.3.179. [DOI] [PubMed] [Google Scholar]
- LaBella F. S., Vivian S., Bindler E. Amino acid composition of neurohypophysial secretory granules and Van Dyke protein. Biochem Pharmacol. 1967 Jun;16(6):1126–1130. doi: 10.1016/0006-2952(67)90288-2. [DOI] [PubMed] [Google Scholar]
- MUNSICK R. A. Effect of magnesium ion on the response of the rat uterus to neurohypophysial hormones and analogues. Endocrinology. 1960 Mar;6:451–457. doi: 10.1210/endo-66-3-451. [DOI] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K. A., Ericsson L. H., Neurath H. Bovine carboxypeptidase A variants resulting from allelomorphism. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1339–1344. doi: 10.1073/pnas.56.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]




