Abstract
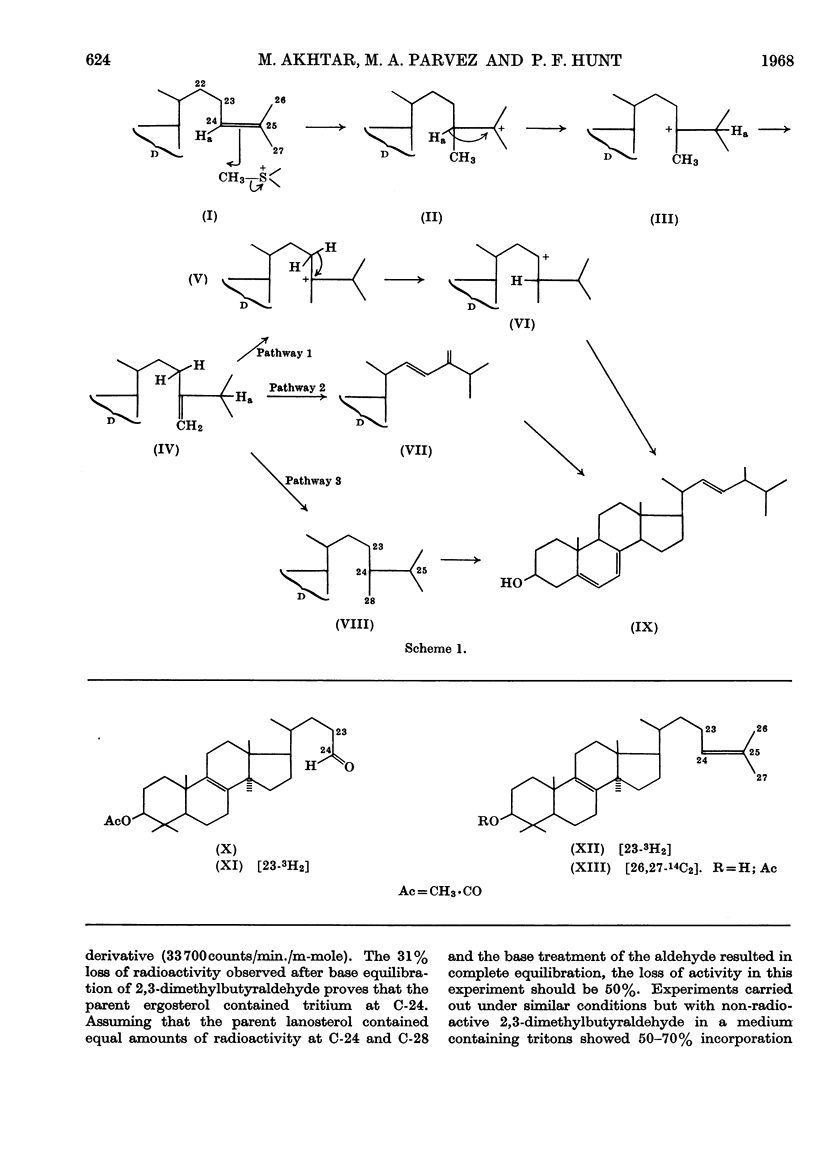
Methods for the chemical synthesis of [23-3H2]lanosterol, [23,25-3H3]24-methyldihydrolanosterol and [24,28-3H2]24-methyldihydrolanosterol are described. It is shown that, in the biosynthesis of ergosterol from [26,27-14C2,23-3H2]lanosterol by the whole cells of Saccharomyces cerevisiae, one of the original C-23 hydrogen atoms is lost and the other is retained at C-23 of ergosterol. It is also shown that 24-methyldihydrolanosterol is converted into ergosterol in good yield and without prior conversion into a 24-methylene derivative. On the basis of these results possible pathways for the formation of the ergosterol side chain from a 24-methylene side chain are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar M., Hunt P. F., Parvez M. A. The transfer of hydrogen from C-24 to C-25 in ergosterol biosynthesis. Biochem J. 1967 Jun;103(3):616–622. doi: 10.1042/bj1030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M., Marsh S. The stereochemistry of the hydrogen elimination in the biological conversion of cholest-7-en-3-beta-ol into cholesterol. Biochem J. 1967 Feb;102(2):462–467. doi: 10.1042/bj1020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhurst S. M., Akhtar M. The conversion of cholest-7-en-3beta-ol into cholesterol. General comments on the mechanism of the introduction of double bonds in enzymic reactions. Biochem J. 1967 Dec;105(3):1187–1194. doi: 10.1042/bj1051187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki H., Bloch K. Studies on the biosynthesis of ergosterol in yeast. Formation of methylated intermediates. J Biol Chem. 1967 Jan 25;242(2):222–227. [PubMed] [Google Scholar]
- Stone K. J., Hemming F. W. The stereochemistry of hexahydroprenol, ubiquinone and ergosterol biosynthesis in the mycelium of Aspergillus fumigatus Fresenius. Biochem J. 1967 Jul;104(1):43–56. doi: 10.1042/bj1040043. [DOI] [PMC free article] [PubMed] [Google Scholar]


