Recent advances highlight accelerated glucose metabolism as one of the hallmarks of cancer cells. Normal differentiated cells usually utilize the process of mitochondrial oxidative phosphorylation to metabolize glucose into carbon dioxide (CO2) and adenosine triphosphate (ATP). However, the cancer cell preferentially takes advantage of aerobic glycolysis to generate lactate and ATP to support the high energy demand for rapid cancer cell proliferation even in the presence of sufficient oxygen.1 This intriguing observation was first reported by the German physiologist Otto Warburg, and thus, this process is termed “the Warburg effect”.2 As a frequent cellular event in tumor cells, accelerated glucose metabolism can provide several metabolite precursors of macromolecule and remodel the tumor microenvironment by its metabolic end product lactate. As a unique characteristic of tumor cells, the enhanced aerobic glycolysis in cancer cells has been widely used to distinguish cancer cells from normal cells in the 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (18F-FDG-PET/CT) medical examination.2
In spite of the pathological importance of aerobic glycolysis “the Warburg effect” in cancer, the disruption and consequence of the abnormalities in other glucose-related metabolic pathways are poorly understood in cancer. One such pathway is glycogen metabolism.3 A recent study published in Cell by Prof. Dawang Zhou's team4 from Xiamen University reported a previously unknown function of glycogen-dependent hepatocarcinogenesis in the early stages of liver cancer. Prof. Zhou's team reported how the accumulation of glycogen drives hepatocarcinogenesis by targeting the Hippo signaling pathway in a phase separation-dependent manner, which provides novel mechanistic insights into the previously unknown function of glycogen storage in the initiation of hepatic malignancies. This study suggests that accumulated glycogen serves as a biomarker for the early detection of patients with liver cancer.
Glycogen is a soluble macromolecule in the cytoplasm and a predominant storage form of glucose. Glycogen is predominantly produced and stored in the hepatocytes and muscle cells. It is comprised of many glucose residues that are regularly assembled into a macromolecule with long branched units of glucose units. The glycogen synthesis and breakdown are tightly modulated by the human body to maintain a stable level of blood glucose. According to genetic studies, it is known that mutated enzymes that participate in glycogen metabolism may engender glycogen storage diseases (GSDs). Patients with GSDs experience some general symptoms, such as retarded growth, heat intolerance, hypoglycemia, hyperlipidemia and hepatomegaly. Among these candidate enzymes, the mutation of glucose-6-phosphatase (G6PC) gene results in not only hepatomegaly but also hepatocellular adenomas and carcinomas. It is known that G6PC mainly catalyzes the conversion from glucose-6-phosphate (G6P) toward glucose. Furthermore, defects in the G6PC gene would trigger the excessive accumulation of glycogen in the liver, leading to hepatomegaly and an increased risk of liver cancer.5 Some patients with G6PC mutation died in childhood due to the disease progression. However, the underlying molecular mechanisms are poorly explored at the current stage.
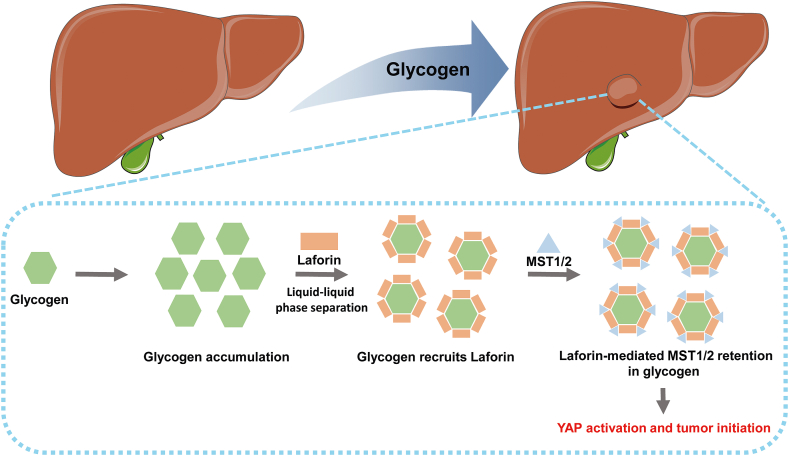
According to a recent study, Prof. Zhou's team,4 observed that elevated glycogen accumulation occurs in the early malignant human hepatic tissues as well as the early stage of mouse liver tumors (Fig. 1). They also found that G6PC expression was significantly reduced in the premalignant lesions compared with that in the adjacent normal tissues, which may account for the glycogen accumulation in liver tumors. To investigate the functional consequence of G6PC downregulation in hepatocarcinogenesis, the authors established hepatocyte-specific G6PC-KO mice. The G6PC-KO mice displayed hepatomegaly as human patients with G6PC mutation did. As the liver size is tightly regulated by the Hippo signaling pathway, the authors then evaluated the expression profiles of the Hippo signaling pathway in liver cancer.
Fig. 1.
Schematic of the regulation of Hippo signaling pathway by hepatic glycogen storage. Abbreviations: MST1/2, Mammalian sterile 20-like kinase 1 and 2; YAP, Yes-associated protein.
The Hippo pathway was first identified in Drosophila as an important regulator of organ size, which was subsequently found to be highly conserved across different species.6 In the past decade, growing evidence indicates that the Hippo signaling pathway orchestrates a wide spectrum of hepatic cellular events, including cell fate decisions, liver regeneration, and hepatocarcinogenesis.7 The disruption of the Hippo signaling pathway would initiate various chronic liver diseases. The Hippo signaling pathway is tightly modulated by several protein kinases, such as mammalian sterile 20-like kinase 1 and 2 (MST1/2) and large tumor suppressor 1 and 2 (LATS1/2).8 The serine-threonine protein kinases MST1 and MST2 form a complex with the scaffold protein SAV1. The MST1/2 complex then phosphorylates and activates the serine-threonine protein kinases LATS1 and LATS2. LATS1 and LATS2, together with the regulatory proteins MOB kinase activator 1A and 1B (MOB1A and MOB1B), phosphorylate Yes-associated protein (YAP) and its paralogue protein tafazzin (TAZ). The phosphorylation of YAP/TAZ complex is then targeted by the ubiquitin ligase beta-transducin repeat-containing protein (β-TrCP), leading the proteasomal degradation. When the MST1/2 or LATS1/2 complex is suppressed by extracellular signals, the YAP/TAZ complex is hypophosphorylated and translocates to the nucleus, leading to the transcriptional activation of downstream target genes. Thus, the MST1/2 or LATS1/2 complex functions as an important negative regulator to suppress the activity of the YAP/TAZ complex. Recent investigations found that YAP is highly expressed in several cancer types, including colorectal cancer, breast cancer, lung cancer, cholangiocarcinoma, and hepatocellular carcinoma. The enhanced YAP expression or deletion of MST1/2 induces uncontrolled tumor growth in the liver,9 suggesting that YAP serves as a driver gene in hepatocarcinogenesis.
In the study of Prof Zhou's team, loss of G6PC in hepatocytes led to significantly reduced MST1/2 activities and constitutively active YAP, as indicated by a decreased YAP phosphorylation level and increased YAP nuclear localization. Intriguingly, they also found that MST1/2 co-localizes with glycogen foci in the mouse hepatocytes. Subsequent investigators demonstrated that accumulated glycogen underwent liquid-liquid phase separation and sequestrated MST1 and MST2 by interacting with glycogen-binding protein laforin. Recent studies indicate that the alteration of liquid-liquid phase separation reshapes the biochemical landscape of cancer cells.10 As the MST1/2 complex is hijacked by glycogen droplets, the YAP escapes from MST1/2-mediated phosphorylation and hypophosphorylated YAP translocates to the nucleus, leading to the activation of gene transcription and uncontrolled cell proliferation. Taken together, this finding indicated that the elevated glycogen accumulation may lead to an increased risk of liver cancer in a YAP-dependent manner in multiple genetic mouse models, suggesting that glycogen droplets within hepatocytes have a previously unknown role in initiating hepatocarcinogenesis through YAP activation.
Based on the current study, some open questions are raised. For instance, it is largely unknown how glycogen liquid-like droplets are condensed during hepatocarcinogenesis. Is it possible that researchers can develop novel therapeutic approaches or diagnostic tools by targeting glycogen accumulation? Do any biological mechanical sensors participate in this process? In addition, the authors reported that a non-metabolic function of glycogen is promoting cellular transformation. It remains poorly understood how glycogen accumulation accelerates tumorigenesis in a metabolic-dependent manner. Thus, further explorations are needed to fully address the underlying molecular mechanisms.
Authors’ contributions
Q. Zhang and W. Liang wrote the manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This manuscript is supported by grant funded by the National Natural Science Foundation of China (Grant No. 81902886).
Footnotes
Edited by Peiling Zhu and Genshu Wang.
Contributor Information
Weicheng Liang, Email: liangwch5@mail.sysu.edu.cn.
Qi Zhang, Email: keekee77@126.com.
References
- 1.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer. 2016;16:635–649. doi: 10.1038/nrc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritterson Lew C, Guin S, Theodorescu D. Targeting glycogen metabolism in bladder cancer. Nat Rev Urol. 2015;12:383–391. doi: 10.1038/nrurol.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Q, Li J, Zhang W, et al. Glycogen accumulation and phase separation drives liver tumor initiation. Cell. 2021;184:5559–5576. doi: 10.1016/j.cell.2021.10.001. e19. [DOI] [PubMed] [Google Scholar]
- 5.Chou JY, Jun HS, Mansfield BC. Glycogen storage disease type I and G6Pase-β deficiency: etiology and therapy. Nat Rev Endocrinol. 2010;6:676–688. doi: 10.1038/nrendo.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell JO, Camargo FD. Hippo signalling in the liver: role in development, regeneration and disease. Nat Rev Gastroenterol Hepatol. 2022;19:297–312. doi: 10.1038/s41575-021-00571-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tordjmann T. Hippo signalling: liver size regulation and beyond. Clin Res Hepatol Gastroenterol. 2011;35:344–346. doi: 10.1016/j.clin. [DOI] [PubMed] [Google Scholar]
- 9.Lu L, Li Y, Kim SM, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci USA. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta S, Zhang J. Liquid-liquid phase separation drives cellular function and dysfunction in cancer. Nat Rev Cancer. 2022;22:239–252. doi: 10.1038/s41568-022-00444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]