Abstract
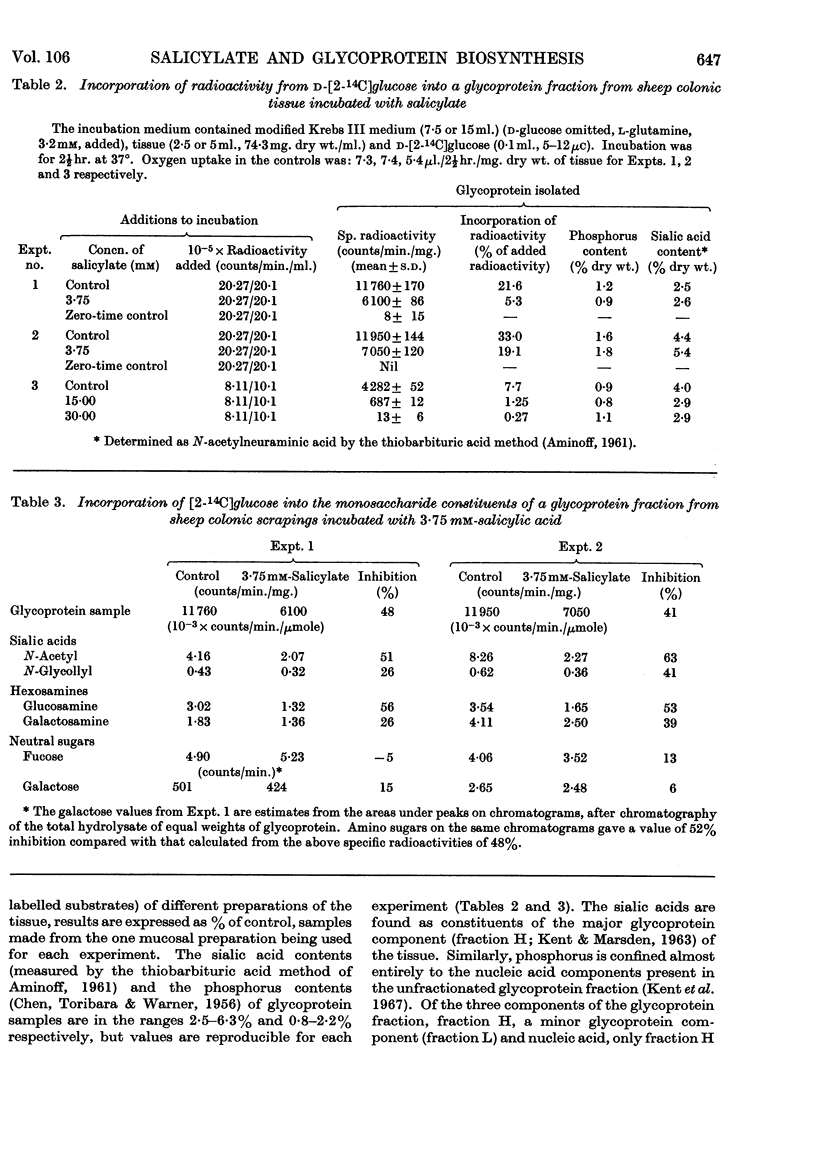
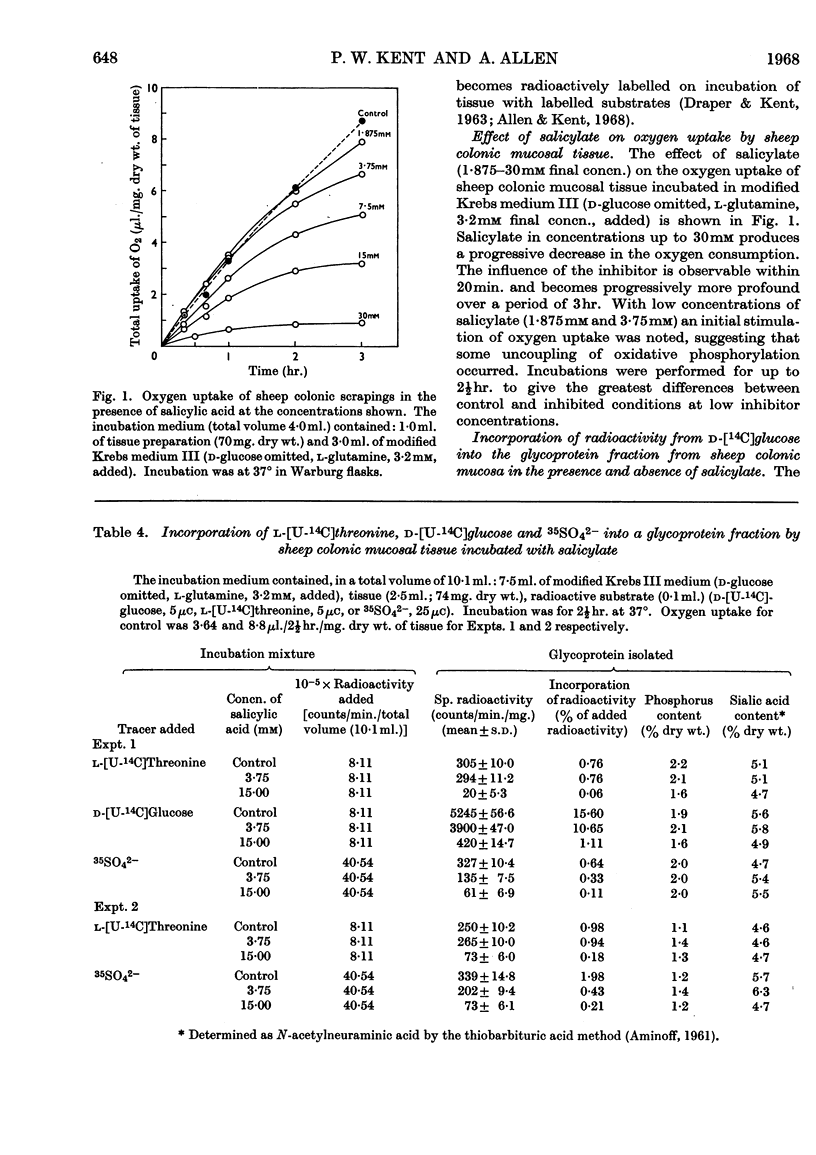
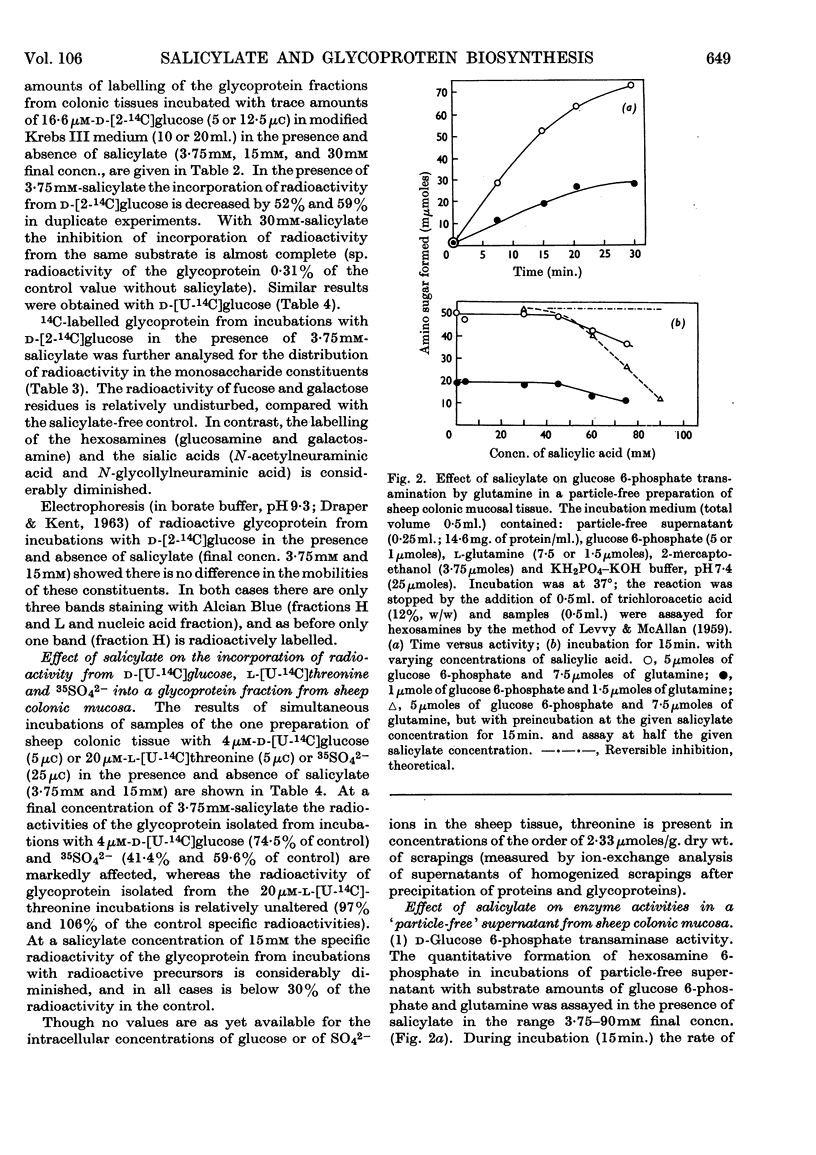
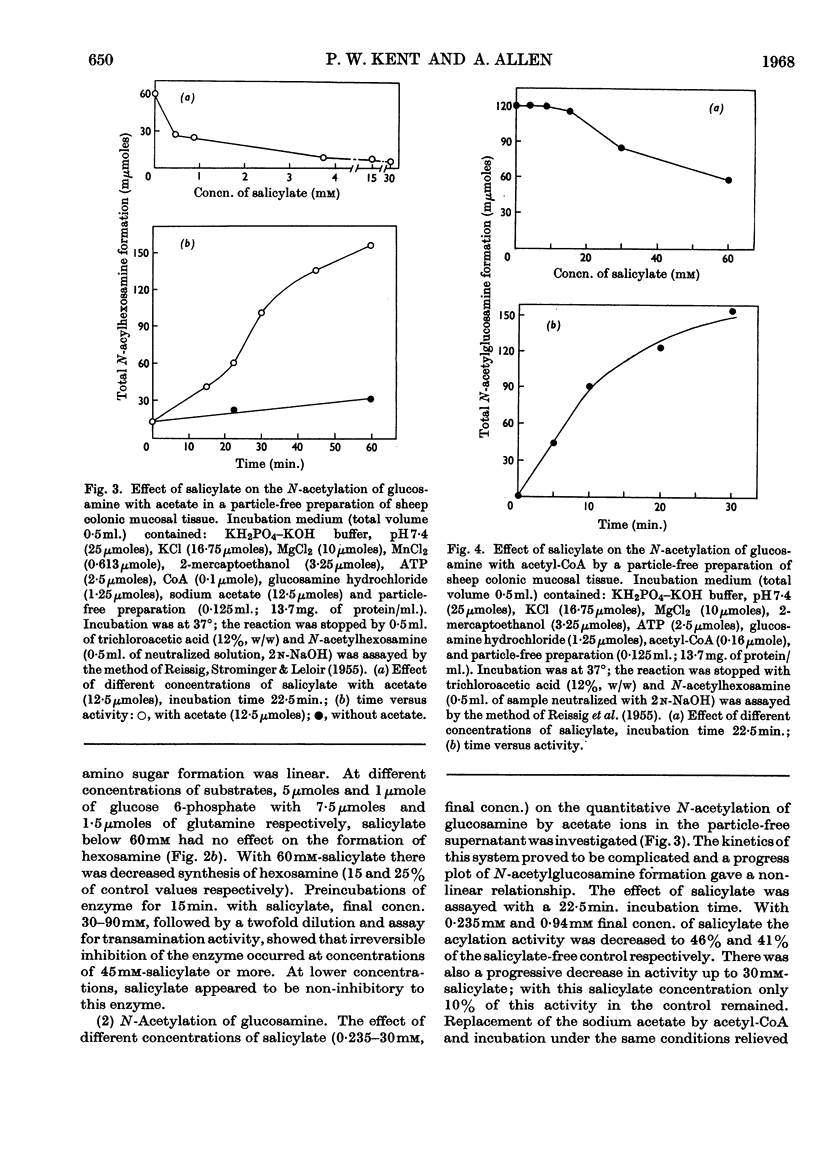
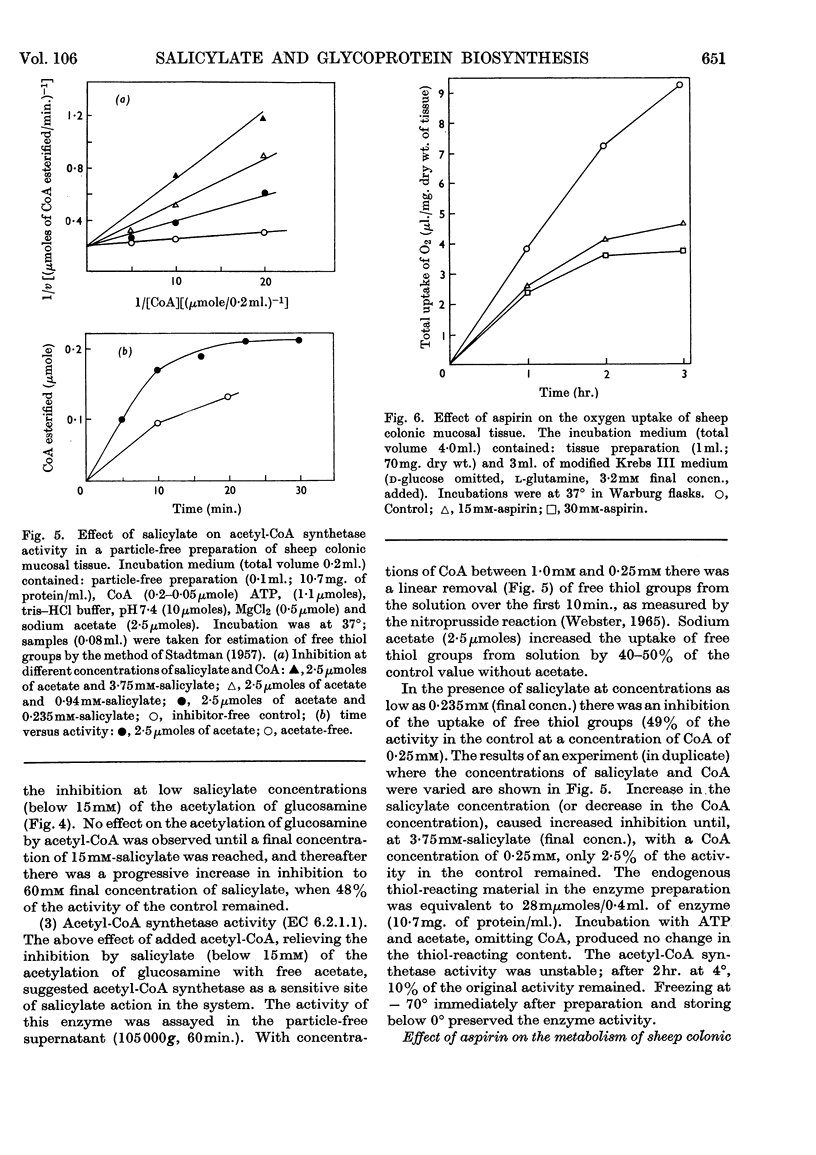
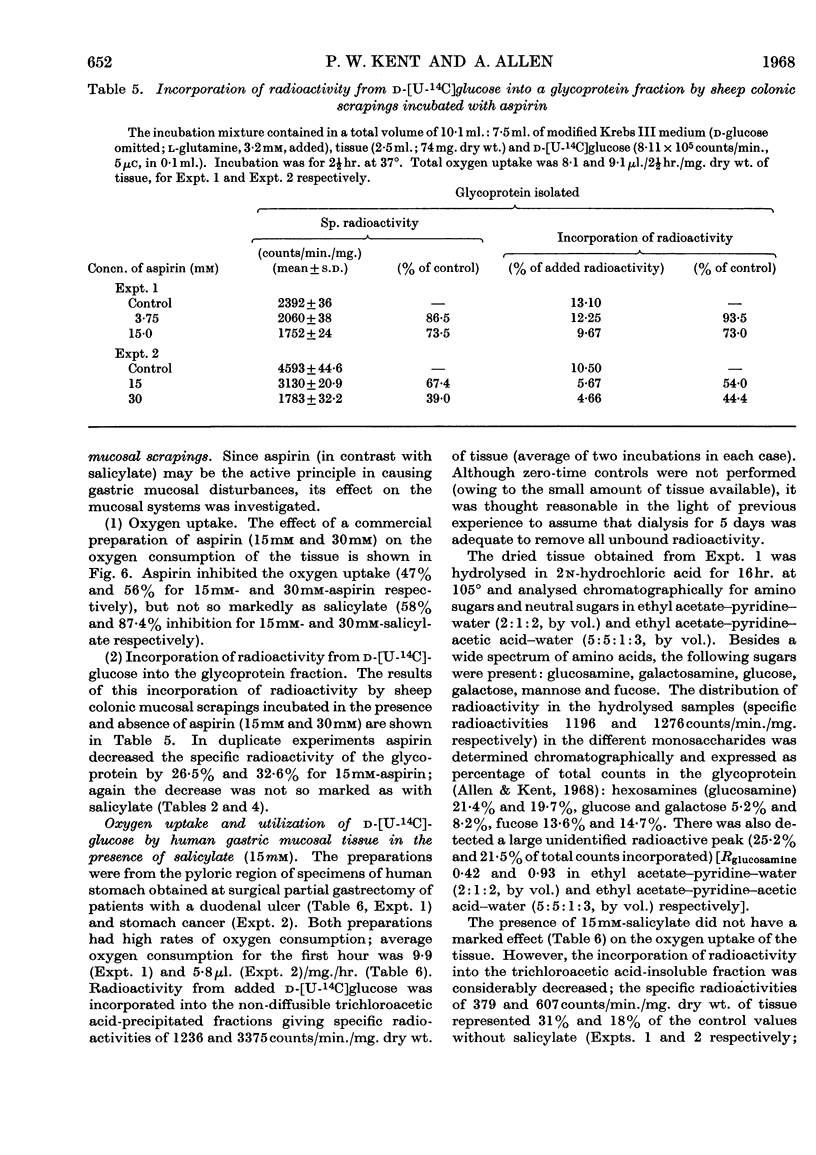
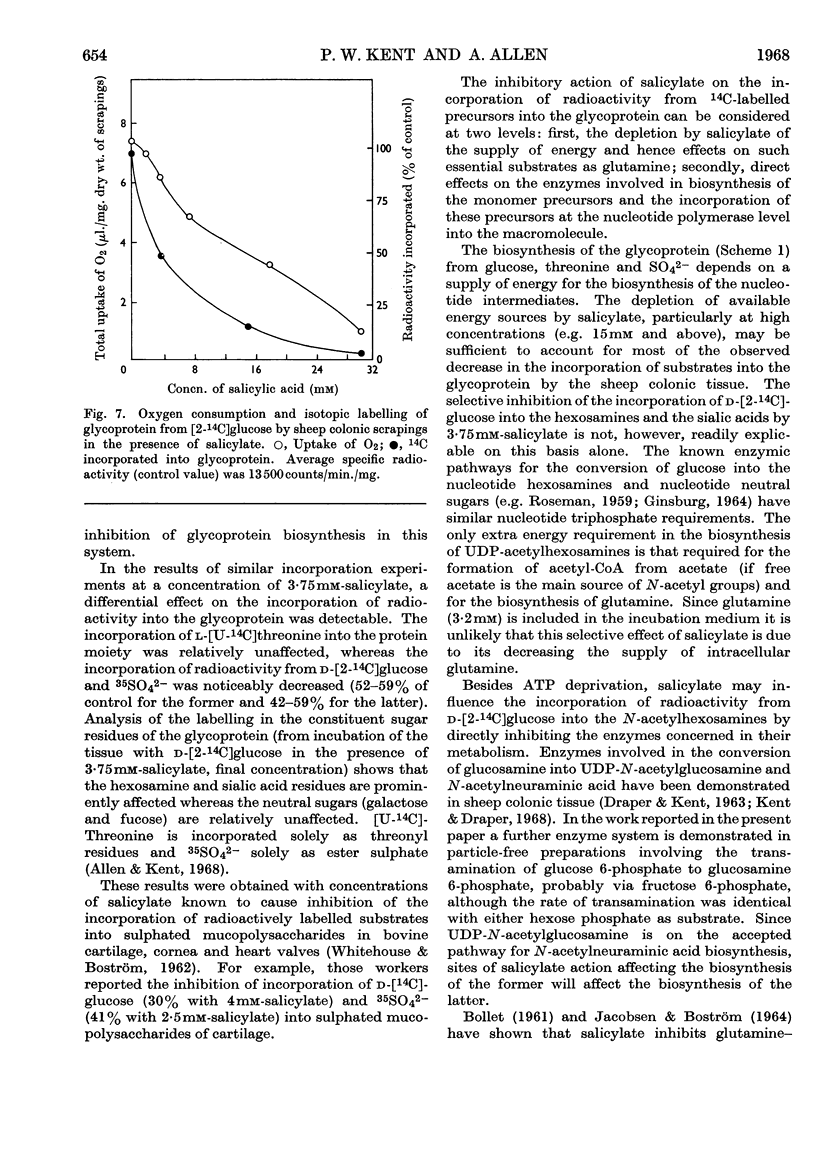
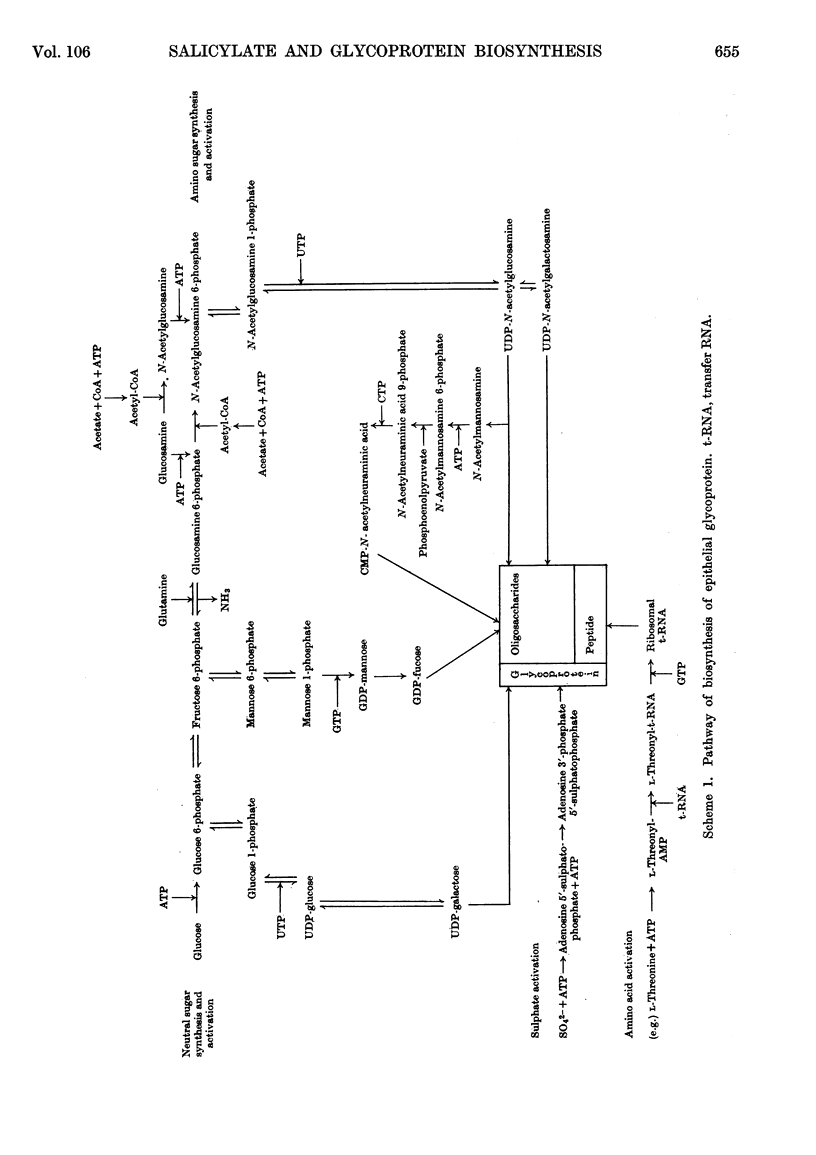
1. Incubation of sheep colonic mucosal scrapings in Krebs–Ringer buffer for 2½hr. in the presence of salicylate (15mm) resulted in decreased incorporation of radioactivity into the epithelial glycoprotein from the following labelled precursors: 16·6μm-d-[2-14C]glucose (83·9% inhibition), 20μm-l-[U-14C]threonine (82%) and 35SO42−(79%). Oxygen uptake measured simultaneously was diminished to 41% of the control value. 2. At lower concentrations of salicylate (e.g. 3·75mm), incorporation of 20μm-l-[U-14C]threonine was little affected (3–6% inhibition), whereas utilization of 4μm-d-[U-14C]glucose and 35SO42− was inhibited (41–48% and 40–59% of the control values respectively). 3. Analysis of the papain-digested glycoprotein from tissue incubations with 16·6μm-d-[2-14C]glucose in the presence of salicylate (3·75mm) showed large decreases in labelling of N-acetylneuraminic acid and N-glycollylneuraminic acid residues (57% and 34% of the control values respectively) and of hexosamine constituents (glucosamine, 55% inhibition; galactosamine, 33% inhibition). Labelling of neutral sugars (galactose and fucose) was relatively little affected (9 and 11% inhibition respectively). 4. Glucose 6-phosphate transaminase and glucosamine 6-phosphate acetylase in particle-free enzyme preparations of the sheep tissue were unaffected by salicylate at the above concentrations. Acetyl-CoA synthetase was markedly inhibited. 5. Human gastric mucosa (from operation), on incubation as above, had in one experiment an oxygen consumption of 9·9μl./hr./mg. dry wt. of tissue and incorporated 5μm-d-[U-14C]glucose (15·8% of the total radioactivity added) into bound hexosamine (20·6% of the total radioactivity incorporated), hexoses (glucose and galactose, 5·7%) and fucose (14·2%). The presence of salicylate (15mm) decreased the incorporation of 5μm-d-[U-14C]glucose into the glycoprotein by 74%, all sugar constituents being affected, without influence on the rate of oxygen consumption. 6. The results suggest an inhibitory effect of salicylate on glycoprotein biosynthesis at the level of the amino sugar intermediates.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON K. W. STUDIES ON THE BIOCHEMICAL MECHANISM OF THE GASTRIC EROSION CAUSED BY ASPIRIN. Biochem Pharmacol. 1964 Nov;13:1513–1517. doi: 10.1016/0006-2952(64)90202-3. [DOI] [PubMed] [Google Scholar]
- ANNISON E. F., LENG R. A., LINDSAY D. B., WHITE R. R. THE METABOLISM OF ACETIC ACID, PROPIONIC ACID AND BUTYRIC ACID IN SHEEP. Biochem J. 1963 Aug;88:248–252. doi: 10.1042/bj0880248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A., Kent P. W. Biosynthesis of intestinal mucins. Effect of puromycin on mucoprotein biosynthesis by sheep colonic mucosal tissue. Biochem J. 1968 Jan;106(1):301–309. doi: 10.1042/bj1060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLLET A. J. Inhibition of glucosamine-6-PO4 synthesis by salicylate and other anti-inflammatory agents in vitro. Arthritis Rheum. 1961 Dec;4:624–631. doi: 10.1002/art.1780040608. [DOI] [PubMed] [Google Scholar]
- BRODY T. M. Action of sodium salicylate and related compounds on tissue metabolism in vitro. J Pharmacol Exp Ther. 1956 May;117(1):39–51. [PubMed] [Google Scholar]
- DAVIDSON E. A., BLUMENTHAL H. J., ROSEMAN S. Glucosamine metabolism. II. Studies on glucosamine 6-phosphate N-acetylase. J Biol Chem. 1957 May;226(1):125–133. [PubMed] [Google Scholar]
- Draper P., Kent P. W. Biosynthesis of intestinal mucins. 4. Utilization of [1-C]glucose by sheep colonic mucosa in vitro. Biochem J. 1963 Feb;86(2):248–254. doi: 10.1042/bj0860248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBURG V. SUGAR NUCLEOTIDES AND THE SYNTHESIS OF CARBOHYDRATES. Adv Enzymol Relat Areas Mol Biol. 1964;26:35–88. doi: 10.1002/9780470122716.ch2. [DOI] [PubMed] [Google Scholar]
- HINES W. J., BRYANT C., SMITH M. J. EFFECTS OF SALICYLATE, GAMMA-RESORCYLATE AND GENTISATE ON OXIDASE ENZYME SYSTEMS FROM GUINEA-PIG LIVER MITOCHONDRIA. Biochem Pharmacol. 1963 Oct;12:1109–1116. doi: 10.1016/0006-2952(63)90085-6. [DOI] [PubMed] [Google Scholar]
- JACOBSON B., BOSTROEM H. STUDIES ON THE BIOCHEMISTRY OF HEART VALVES. II. THE EFFECT OF AGING AND ANTI-INFLAMMATORY DRUGS ON THE SYNTHESIS OF GLUCOSAMINE 6-PHOSPHATE AND PHOSPHOADENOSINE PHOSPHOSULFATE BY BOVINE HEART VALVES. Biochim Biophys Acta. 1964 Jul 7;83:152–164. doi: 10.1016/0926-6526(64)90031-x. [DOI] [PubMed] [Google Scholar]
- KENT P. W., PASTERNAK C. A. Biosynthesis of intestinal mucins. 3. Formation of active sulphate by cell-free extracts of sheep colonic mucosa. Biochem J. 1958 Jul;69(3):453–458. doi: 10.1042/bj0690453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KODICEK E., LOEWI G. The uptake of (35S) sulphate by mucopolysaccharides of granulation tissue. Proc R Soc Lond B Biol Sci. 1955 Aug 16;144(914):100–115. doi: 10.1098/rspb.1955.0037. [DOI] [PubMed] [Google Scholar]
- Kent P. W., Draper P. Biosynthesis of intestinal mucins. Sialic acids of sheep colonic epithelial mucin. Biochem J. 1968 Jan;106(1):293–299. doi: 10.1042/bj1060293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVVY G. A., MCALLAN A. The N-acetylation and estimation of hexosamines. Biochem J. 1959 Sep;73:127–132. doi: 10.1042/bj0730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN W. T., WATKINS W. M. Some aspects of the biochemistry of the human blood-group substances. Br Med Bull. 1959 May;15(2):109–113. doi: 10.1093/oxfordjournals.bmb.a069732. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- ROSEMAN S. Metabolism of connective tissue. Annu Rev Biochem. 1959;28:545–578. doi: 10.1146/annurev.bi.28.070159.002553. [DOI] [PubMed] [Google Scholar]
- WHITEHOUSE M. W., BOSTROM H. Studies on the action of some anti-inflammatory agents in inhibiting the biosynthesis of mucopolysaccharide sulphates. Biochem Pharmacol. 1961 Jul;7:135–150. doi: 10.1016/0006-2952(61)90150-2. [DOI] [PubMed] [Google Scholar]
- WHITEHOUSE M. W., BOSTROM H. The effect of some anti-inflammatory (anti-rheumatic) drugs on the metabolism of connective tissues. Biochem Pharmacol. 1962 Dec;11:1175–1201. doi: 10.1016/0006-2952(62)90196-x. [DOI] [PubMed] [Google Scholar]
- WHITEHOUSE M. W., LASH J. W. Effect of cortisone and related compounds on the biogenesis of cartilage. Nature. 1961 Jan 7;189:37–39. doi: 10.1038/189037a0. [DOI] [PubMed] [Google Scholar]
- Webster L. T., Jr Studies of the acetyl coenzyme A synthetase reaction. II. Crystalline acetyl coenzyme A synthetase. J Biol Chem. 1965 Nov;240(11):4158–4163. [PubMed] [Google Scholar]
- Webster L. T., Jr Studies of the acetyl coenzyme A synthetase reaction. V. The requirement for monovalent and divalent cations in partial reactions involving enzyme-bound acetyl adenylate. J Biol Chem. 1967 Mar 25;242(6):1232–1240. [PubMed] [Google Scholar]
- Whitehouse M. W., Skidmore I. F. Concerning the regulation of some diverse biochemical reactions, underlying the inflammatory response, by salicylic acid, phenylbutazone and other acidic antirheumatic drugs. J Pharm Pharmacol. 1965 Oct;17(10):668–671. doi: 10.1111/j.2042-7158.1965.tb07583.x. [DOI] [PubMed] [Google Scholar]


