Abstract
Infection with hepatitis B virus (HBV) is one of the significant challenges worldwide. Despite the availability of antiviral drugs against this virus, the most critical strategy to prevent HBV infection is HB vaccination. Basically, despite widespread conventional HB vaccination, due to various reasons, including waning of hepatitis B surface antibody (HBsAb) titer after vaccination, the emergence of vaccine-escape mutants, failure to respond to the vaccine due to viral and host factors, levels of response in high-risk individuals and non-responders to conventional HB vaccination remains a major, unsolved and severe concern. This review focuses on the underlying reasons for conventional hepatitis B vaccination failures. It also suggests solutions to overcome these failures by highlighting significant advances in vaccination, including hepatitis B third-generation vaccines and adjuvanted hepatitis B vaccines as efficient alternatives to second-generation vaccines. Potentially, these new strategies will compensate for the shortcomings caused by second-generation vaccines. Adherence to these denouements has a significant role in preventing the circulation of HBV among individuals and reducing the global burden of HBV-related diseases.
Keywords: Hepatitis B virus, Hepatocellular carcinoma, Vaccination, Hepatitis B third-generation vaccines
Introduction
Infection with hepatitis B virus (HBV) remains a significant public health issue worldwide. According to the latest reports published in 2019, approximately 296 million individuals are struggling with chronic hepatitis B infection, with about 1.5 million new cases diagnosed annually [1–3]. Subsequently, chronic and acute infection caused by this virus causes severe damage to the liver, which following chronic infection for a very long time and gradually causes irreversible consequences such as cirrhosis and hepatocellular carcinoma (HCC) [2, 4]. Despite the availability of widespread antiviral treatments, these antiviral drugs are not able to perfectly clear virus inside the nucleus; hence, the best method to control the infection is through vaccination [5]. However, still, HBV infection following vaccination has become a considerable public health concern. Moreover, the lack of antibodies in immunocompromised individuals and the emergence of vaccine-escape mutants lead to inadequate response to vaccination. The present review focuses on the complicated problems following effective HBV vaccination, highlighting the reasons for the emergence of infection. Also, strategies such as the use of booster doses, third-generation HB vaccines and new adjuvanted vaccines are suggested as either the best alternative to conventional HB vaccines or as additives to previous vaccinations (regarding boosters) (Table 1).
Table 1.
Development of hepatitis B vaccines to date
| Types of HBV vaccines | Year | Generation | Preparation process |
|---|---|---|---|
| Plasma-derived HBV vaccines | 1981 | First-generation HBV vaccine | Purification of HBsAg in plasma obtained from HBsAg carriers |
| HBV DNA recombinant vaccine | 1986 | Second-generation HBV vaccines | Expression of the HBsAg in yeast cells |
| Expression of pre-S/S2 in mammalian cells | 1990 | Third-generation HBV vaccines | Pre-S/S2 or S, pre-S1, pre-S2 expression in mouse/CHO cell lines |
CHO Chinese hamster ovary
A brief overview of hepatitis B vaccines
Plasma-derived vaccines
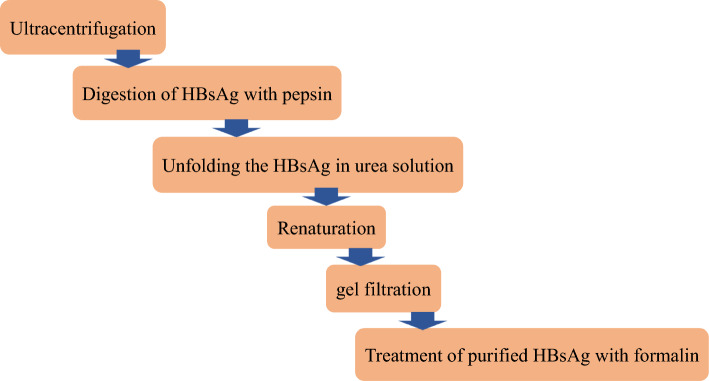
Following the discovery of the Australian antigen and the observation of Dane particles using an electron microscope, progress in the production of the hepatitis B vaccine has been significant [6, 7]. Since there is no successful in vitro cell culture for HBV replication, it seems unlikely to produce hepatitis B vaccine by cell culture techniques. The development of the hepatitis B vaccine was based on Dr Krugman’s efforts and findings. In this line, significant progress was made in the production of hepatitis B vaccine by eliminating the infectivity of this virus by boiling the plasma of HBV carriers. After receiving the boiled plasma from infected individuals, the production of anti-HBs antibodies against HBsAg was induced, followed by procedures which resulted in partial protection against the virus [8, 9]. In the HBV carriers, HBV antigens are produced entirely naturally, which provides the prospect of making a hepatitis B vaccine [7]. Considering the susceptibility of chimpanzees to human HBV infection, it was possible to introduce a suitable animal model to assess the safety and efficacy of hepatitis B vaccine [10]. HBV challenge showed the protection of vaccinated chimpanzees by the purified HBsAg [11]. Therefore, in the early 1980s, in countries such as the USA and France, using 22 nm hepatitis B surface antigen (HBsAg) collected and obtained from HBsAg carriers, so-called plasma-derived vaccines were made as the first-generation vaccines against hepatitis B. Purification and inactivation of the first-generation vaccines was done through treatments with heat, formaldehyde, pepsin and urea. Following vaccination with plasma-derived vaccines, millions of individuals have proven satisfactory protection with adequate effectiveness and safety [12, 13] (Fig. 1).
Fig. 1.
Production steps of the first generation of HB vaccines
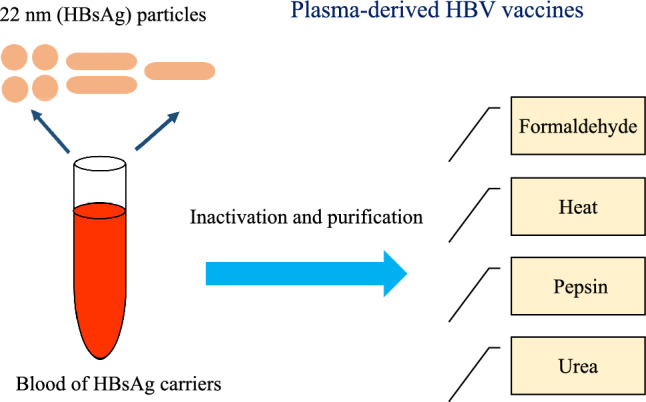
Since 1982, plasma-derived vaccines for HBV were introduced as the first commercially accessible hepatitis B vaccines. They are produced from the inactivated plasma fluid of chronically asymptomatic donors infected with HBV through the collection of hepatitis B surface antigen (HBsAg) subviral particles (SVP) [9, 14]. Following assessment of the success rate of plasma-derived vaccines in millions of people, the first licensed vaccines against HBV were produced for the immunization program in high-risk individuals [15]. The high efficiency and safety of the hepatitis B vaccine in the prevention of asymptomatic infection, acute hepatitis B and chronic HBV carriers have been confirmed by comprehensive studies [12, 16, 17]. Subsequently, the first commercial plasma hepatitis B vaccines, plasma-derived vaccines, were licensed in the USA and in France in 1981 and 1982, respectively [18, 19]. Due to the common “antigenic determinant” presence in isolates of HBV and the presence of HBV in different serotypes, the plasma hepatitis B vaccine was designed to be subtype cross-protective [20]. Considering that the primary source of the plasma-derived vaccine was the plasma of the HBV carriers, there was a possibility for co-infection with various pathogens, including HIV. Therefore, in order to eliminate the contamination of all possible viruses and pathogens, various steps were used in the preparation of the HB first-generation vaccine [21] (Fig. 2).
Fig. 2.
Steps to remove possible pathogens in plasma-derived hepatitis B vaccine preparation
On the other hand, in North America and Europe, concerns have been raised about the possibility of vaccine plasma contamination with other viruses, including HIV. Based on the findings, these issues were completely unfounded as no contamination with HIV or other pathogens was reported. However, considerations about the possible safety consequences of plasma-derived vaccines eventually stopped the use of first-generation vaccines. Following these challenges, in the mid-1980s, recombinant DNA vaccines were replaced the plasma-derived vaccines.
Second-generation recombinant HB vaccine
Second-generation recombinant vaccines were produced, which were progressed as an alternative to plasma-derived vaccines. Large-scale recombinant vaccine production was performed by expressing HBsAg in Saccharomyces cerevisiae and subsequently in mammalian cells [5, 15, 22, 23]. Today, worldwide, the current hepatitis B vaccines in use are entirely of recombinant types [5]. Several steps are required to prepare recombinant vaccines. S gene is isolated from HBV and inserted in yeast cells. Then, during the fermentation process, multitude of HBsAg is produced. The next steps include extraction and purification. Then, to increase the immunogenicity of the HBV vaccine, HBsAg is absorbed on aluminum hydroxide (adjuvant) [5, 24–26] (Fig. 3).
Fig. 3.
Various steps of second-generation HB vaccines preparation
Third-generation HB vaccines
Despite the safety and immunogenicity of second-generation vaccines, post-translational modifications in a fungal system were still a cause of concern. Therefore, mammalian cells, including mouse-derived cell lines, as well as Chinese hamster ovary (CHO) have been used in the production of third-generation HB vaccines. These cells are able to express and secrete two glycoproteins: small (S), middle (pre-S2/S) or three envelope proteins: large (pre-S1/S2/S) HBsAg. France, Germany, and Korea were among the countries that produced third-generation vaccines by transfecting HBV envelope proteins in mammalian cells [27–29]. Findings have shown that HB vaccines, including pre-S/S viral proteins, are very effective in individuals who are poor responders or non-responders to conventional HB vaccines. Third-generation vaccines can induce antibody response faster than recombinant vaccines produced in yeast [30–32]. Efficacy and immunogenicity of third-generation HBV vaccines are shown in Table 7.
Table 7.
Efficacy and immunogenicity of third-generation HBV vaccines
| Type of study | Study subjects | Viral antigens/Proteins | Number of studied subjects | Age | Gender | Number of doses | Seroprotection rate (SPR) | References |
|---|---|---|---|---|---|---|---|---|
| Phase 3 trial, double-blind, randomized, | Adults | S, pre-S1, and pre-S2 antigens | n = 718 | ≥ 18 years | Third vaccination (on days 0, 28, and 168) | 656 (91·4%) | [114] | |
| Phase 3 trial, double-blind, randomized, | Adults | S, pre-S1, and pre-S2 antigens | n = 625 | ≥ 45 years | Third vaccination (on days 0, 28, and 168) | 559 (89·4%) | [114] | |
| Randomized Clinical Trial, Phase 3 | Healthy adults | 3-antigen (3A)-HBV | n = 1753 | 18 to 45 years | Third Injection | 1740 (99.3%) | [115] | |
|
Clinical Trial, Randomized, Phase 3 |
Healthy volunteer | 3-Antigen (Pre-S1/Pre-S2/S) | n = 50 | 18–45 years |
Male [18] Female [32] |
3-dose regimens of 3AV (10 μg) | 100% | [116] |
| Phase IV study | Healthy young adults | 3-Antigen (Pre-S1/Pre-S2/S) | n = 91 | 20–40 years |
Male [74] Female [17] |
Three IM doses of 10 μg (at 0, 1 and 6 months) |
Seroprotective levels: 100% * at month 7: 97.6% (n = 81), high responders (≥ 100 mIU/mL) |
[117] |
| Prospective cohort | HIV-infected adults |
Sci-B-Vac™: mimics 3-Antigen (Pre-S1/Pre-S2/S) |
n = 31 | 10 µg/three dose/intramuscularly/0, 1 and 6 months | 84% | [118] |
HB vaccines using adjuvant
In the era of industrial vaccinology, new adjuvants have been used to increase the immune response against the targeted genes. Apart from routine application in current vaccines, adjuvants are intended for use in vaccines for poor responders and non-responders. An HBsAg adjuvanted vaccine with increased immunogenicity using 3-O-desacyl 1–4′monophosphoril lipid A (MPL) and aluminum phosphate has been prepared. Several countries have used these efficient and successful productive vaccines in liver transplant recipients and in hemodialyzed and pre-hemodialyzed patients [33, 34]. Following the third dose administration among non-responders, the seroprotection rate in hepatitis B vaccine (HBsAg/AS04) and Engerix-B vaccines was reported as 98% and 68%, respectively [35]. Appropriate protection/immune response after adjuvanted AS04C hepatitis B vaccination has been reported in autoimmune disease patients [36]. In diabetics, the HBsAg-1018 hepatitis B vaccine has induced a higher seroprotection rate than the HBsAg-Eng hepatitis B vaccine [37]. After three doses of vaccination with HepB-CpG, seroprotection has occurred in all naïve HIV-infected individuals without a history of hepatitis B vaccination [38]. Moreover, seroconversion has been observed to be three times higher in chronic kidney disease individuals who received adjuvanted hepatitis B vaccine compared to non-adjuvanted hepatitis B vaccines [39]. Among hemodialysis adults who were previously non-responders to HB vaccination, a booster dose of the HepB-CpG hepatitis B vaccine produced a greater seroprotection rate than the HepB-AS04 and HepB-Eng vaccines [40]. Recently, a recombinant HB vaccine with CpG (cytosine phosphate guanosine) adjuvant has been approved by FDA. This vaccine induces innate immunity. Individuals receive two doses of the vaccine within a month. This vaccine has been considered for several advantages, including earlier seroprotection, higher seroprotection rate, a shorter schedule and sensible adherence [41]. Some clinical trial studies in non-responders have reported a greater immunogenic response after receiving adjuvanted vaccines [35, 42]. In addition to using adjuvant 3‐O‐desacyl‐4′‐monophosphoryl lipid A (MPL), the greater antigen content used in the Fendrix® vaccine (40‐µg HBsAg) compared to the Engerix‐B® (20 µg) vaccine has induced a greater seroprotection rate [35]. Among 35 non‐responders, seroprotection rate after the two vaccination doses with Engerix‐B® and Heplisav‐B® was demonstrated 66.7% and 88.9%, respectively [42]. Adjuvants, including aluminum adjuvant, are used in vaccine preparation and play a key role in increasing the humoral immune response following vaccination (Table 2). Recently, a novel PF3 nano-adjuvant has been designed to improve the humoral and cellular immune response after hepatitis B vaccination, covering the deficiency of aluminum adjuvant [43].
Table 2.
Immunogenicity of adjuvanted HB vaccines
| Study type | Adjuvanted hepatitis B vaccine | Studied individuals | Age | Gender | Participant (n) | Number of doses | Seroprotection rate | References |
|---|---|---|---|---|---|---|---|---|
| Single-blind, randomized/trials | HBsAg/AS04 | Healthy non‐responders (majority: healthcare workers) | 20–60 years old | 0, 1, 6 months | 98% | [35] | ||
| Controlled phase 2 trial, randomized, Double‐blinded | HBAI20 vaccine | Healthy non‐responders | 18 to 59 years | Female: 59 (58.4%), Male: 42 (41.6%) | n = 101 | 0, 1 and 2 months (third doses of vaccination) | 80/87 (92.0%) | [44] |
| Controlled phase 2 trial, randomized, Double‐blinded | HBVaxPro®‐10 µg | Healthy non‐responders | 18 to 59 years | Female: 19 (59.4%), Male: 13 (40.6%) | n = 32 | 0, 1 and 2 months (third doses of vaccination) | 23/29 (79.3%) | [44] |
| Open-label study | HBAI20 | Non-responders | 18 to 59 years | Male: 5 (50%) | n = 10 | 3 vaccinations [0, 1 and 6 months] | 90% | [45] |
| Non-randomized trial, open-label | HBV-AS04 | Patients dialysis | 67.4 ± 8.4 |
Male: 149 (60%), Female: 99 (40%) |
n = 248 |
four 20-mcg doses * (n = 217): 0,1,2 and 3 months * (n = 31) 0,1,2 and 6 months |
202/248 (81.5%) | [46] |
| Phase III | HB-AS02 | Healthy adults | Age of cohort was 29.9 (6.03) years | Females (50.4%) | n = 399 |
Two doses: at 0 and 1 month |
* after the first vaccination dose (75.9%) * after two vaccination doses (99.7%) |
[47] |
| Observational study | AS04C | Non-responders |
n = 195 patients * non-responders: 126 (65%) |
four-dose | 73.81% | [48] | ||
| Observational cohort study | HepB-alum | HIV-infected individuals | Median age: 41 years | Male (78%) | n = 59 | 3-dose | 57.6% (seroconversion) | [49] |
| Observational cohort study | HepB-CpG | HIV-infected individuals | Median age: 41 years | Male (78%) | n = 61 | 2-dose | 93.4% (seroconversion) | [49] |
Efficient and effective conventional hepatitis B immunization
According to the report issued by the World Health Organization (WHO), the hepatitis B vaccine can protect about 100% against HBV infection [1]. Following the valid clinical trial conclusions, vaccination has been considered since the 1980s. Previous studies have shown the effectiveness of the HBV vaccine in preventing perinatal transmission of HBV [50, 51]. Effective HBV vaccination has shown a vital impact in decreasing the incidence rate of HBV-related disorders, the mortality rate of hepatitis B and also the hepatitis B carriership. Since 1984, effective vaccination of infants in Taiwan, as an endemic country, has been very successful. With the initiation of the worldwide vaccination plan, the situation of chronic carrier individuals of the HBV and perinatal transmission of the virus has been dramatically dropped. So, the appropriate amount of antibody levels was detected in about 85% of vaccinated babies at 18 months [52, 53]. A study in China investigated the efficacy of the HBV vaccine in preventing vertical transmission in two groups of infants. One group receiving HBIG plus HBV vaccine and another group receiving only HBV vaccine were evaluated. Interestingly, no vertical transmission occurred after follow-up in any study group [54].
Seroprotection failure to conventional HB vaccine
The protective titer in the HBV vaccine is evaluated as a hepatitis B surface antibody (HBsAb) titer > 10 IU/L. However, it is usually unable to provide sufficient protection after exposure to HBV and produces a poor response. Indeed, an adequate and appropriate seroprotective response has the potential to induce HBsAb titer ≥ 100 IU/L (Table 3). Despite the administration of three doses of HBV vaccine, an induction titer < 10 mIU/mL indicates non-responders [55, 56].
Table 3.
Anti-HBs titers and response to vaccine definition
| Anti-HBs titers | Vaccine response status |
|---|---|
| ≥ 10 mIU/mL | Protective |
| < 10 mIU/mL | Non-responders |
| 10–100 mIU/mL | Low responders |
| 100–999 mIU/mL | Good responders |
| ≥ 1000 mIU/mL | High responders |
Today, in spite of available effective HBV vaccines, insufficient immune responses are still observed in certain populations. Therefore, due to the lack of an adequate response, this virus is considered a worldwide cause of concern. For example, people with hepatitis C virus (HCV) infection, human immunodeficiency virus (HIV)-infected individuals, elderly people, smokers, obese people, celiac disease, premature babies, hemodialysis patients, individuals with kidney diseases, chronic liver patients and diabetes mellitus (DM) can be mentioned [57, 58]. A study has shown the association of smoking, obesity and age in individuals with possible HBV vaccination failure [55].
A hepatitis B surface antibody (HBsAb) level of more than 10 IU/L is considered an appropriate and efficient immune response. Hepatitis B virus vaccination aims to induce an adequate host immune response against HBV. Due to the lack of adequate response to the HBV vaccine, especially in people at risk, the rate of infection with the HBV increases as expected. Unfortunately, there is no recommendation for HBV pre-vaccination testing for those at risk. Therefore, in people who do not respond to the vaccine, accurate knowledge of the vaccination history of the virus is not available [58–60]. Babies’ response to the HBV vaccine is completely successful and 100%. On the other hand, they produce HBsAb level > 10 mIU/ml. So, to provide adequate protection in infants, the HBV vaccine without or with hepatitis B immune globulin (HBIG) should be administered approximately 24 h after delivery [61, 62].
Several previous studies have evaluated the response to the HBV vaccine in different populations and separately examined nonresponse among some populations. In one study conducted in vaccinated children, HBsAg was detected at 4.2%, and anti-HBc antibody was identified at 4.8%. Among 165 children who received the HBV vaccine, anti-HBs titer ≥ 10 mIU/ml was reported for 129 (78.2%). Among the 129 vaccinated children, good responders and poor responders to the vaccine were reported in 53 (41.1%) and 76 (58.9%), respectively. Their study showed that although children were vaccinated, a moderate prevalence of HBV infection was due to poor vaccine efficacy in their study population [63]. In low birth weight and preterm infants, the hepatitis B vaccine provides less protection and immunogenicity [64–66]. However, the long-term immunization and protection of infants and children against the HBV vaccine seems to be challenging and controversial.
In a study conducted on dialysis patients receiving the HBV vaccine, the rate of HBV vaccine non-responders was reported as 52.3%. Considerably, HBV vaccine non-responders were older than HBV vaccine responders [67]. Hepatitis B virus (HBV) infection is a life-threatening factor in hemodialysis children with chronic kidney disease. One study evaluated HBV vaccine response rates in hemodialysis children, and 33.8% of these children were HCV antibody positive. The results of their study reported 30% and 70% of children higher than 100 IU/mL (great response level) and ≤ 100 IU/mL (hypo-/non-responders), respectively [68].
Unfortunately, non-responders are significant reservoirs for HBV transmission and are considered HBV carriers. Therefore, despite the availability of a successful HBV vaccine, hepatitis B is still considered a critical problem in various populations.
One of the most important factors influencing the response to the HBV vaccine is the age of individuals. It seems that with increasing age and gradually, the level of antibody produced in response to the HBV vaccine decreases significantly. On the other hand, typically, poor responses are observed in approximately 10% of people [69]. It seems that seroprotection failure and poor responses to individuals to the HBV vaccine can significantly affect the effectiveness of the vaccine. Overall, the possible mechanisms of inadequate HBV vaccine response in different populations are quite different depending on multiple factors and are not comprehensively understood. Therefore, more studies and reviews are needed (Table 4).
Table 4.
Response status to conventional HB vaccine
| Studied subjects | Vaccinated subjects | Age | Gender | Vaccine doses | Non-responders anti-HBs titers < 10 mIU/mL |
Low-responders anti-HBs titers 10–100 mIU/ml |
Good responders > 100 mIU/mL |
References |
|---|---|---|---|---|---|---|---|---|
| Students | n = 1704 | Females: 1033 (60.6%) | Three doses | 270 (15.8%) |
* 10–400 IU/L 987 (57.9%) |
* > 400 IU/L 447 (26.3%) |
[70] | |
| Children | n = 1814 |
Male: 1006 Female: 808 |
Three doses (5 μg) 0, 1 and 6 |
3.1% | 28.9% | [71] | ||
| Children | n = 3752 | 6 years |
Male: 2456 (65.4%) Female: 1302 (34.6%) |
Three doses | 723 (19.3%) | 1939 (51.6%) | 1096 (29.2%) | [72] |
| Healthcare workers | n = 200 | 19 to 52 years old | Three doses | 7 (3.5%) | 22 (11.0%) | 171 (85.5%) | [73] | |
|
Healthcare Workers, Medical students |
n = 340 | 18 to 60 years |
Females: 204 (60%) Males: 136 (40%) |
Three doses | n = 40 |
* > 10 mIU/ml n = 300 |
[74] | |
| Healthcare workers | n = 166 |
Three doses 0, 1 and 6 months |
50 (30%) | 18 (10.8%) | 98 (59.2%) | [75] | ||
| Medical Staff | n = 239 | 20–55 years |
Male: 43 (18%) Female: 196 (82%) |
Three doses | 14 (5.9%) | 37 (15.5%) | 188 (78.6%) | [76] |
| Healthcare workers | n = 652 |
Majority: < 25–39 years old |
Females: 271 (41%) Males: 381(59%) |
Three doses 0, 1 and 6 months |
< 25 years: 23 (9%) 25–34 years: 41 (13.0%) 35–49 years: 19 (26%) ≥ 50 years: 7 (63%) |
*Normal responders: < 25 years: 234 (91%) 25–34 years: 270 (87%) 35–49 years: 54 (74%) ≥ 50 years: 4 (36%) |
[77] | |
| Healthcare workers |
n = 151 HBV-vaccinated subjects = 129 |
20–59 years |
Males: 24 (15.9%) Females: 127 (84.1%) |
26 (17.2%) |
*anti-HBs titer > 10 103 (68.2%) |
[78] | ||
| Medical staff | n = 49 | 20.4% | 34.7% | 44.9% | [79] | |||
| Diabetic children | n = 110 | 2–23 years |
Male: 75 Female: 35 |
Three doses | 46 (41.8%) | [80] | ||
| Children | n = 427 | 6 year old |
Female: 223 Male: 204 |
Three doses | 105 (24.6%) | 181 (42.3%) | 141 (33.1%) | [81] |
Occult hepatitis B infection (OBI) following HB vaccination
Although successful universal immunization of newborns is carried out, surface antigen vaccine-escape mutation and OBI infection are considered to be interfering factors in the eradication of HBV infection [82]. The precise mechanism of OBI is not fully understood. Nevertheless, one of the identified mechanisms of OBI is “a” determinant mutation of HBsAg [83]. Creating a mutation in “a” determinant region due to conformational changes of HBsAg leads to a decrease in HBV diagnostic and, subsequently, serological detection failure [84]. The G145R mutation has been distinguished as the most common and well-known HBsAg mutation, which plays an important role in vaccine escape [84]. A region of surface protein (HBsAg) including major hydrophilic region (MHR) domain consists of 99–169 aa. In addition, “a” determinant is located in the region between 124 and 147 aa. On the other hand, “a” determinant mutations are associated with vaccine-escape mutants, and subsequently, OBI occurs (Fig. 4) [85].
Fig. 4.
Occult hepatitis B virus infection (OBI) associated with “a” determinant mutation
In the 1970s, despite the negative results of serum markers, including HBsAg negative, the possibility of another profile of HBV infection was reported [86, 87]. After the development of advanced molecular techniques with great sensitivity, silent or OBI following HBV infection has been characterized [88, 89]. In fact, in this phase of chronic HBV infection, the viral genome is present in the episomal form of covalently closed circular DNA (cccDNA) with low replication conditions, which challenges the detection of HBV DNA in plasma or serum, and if detectable, a low viral level indicates approximately < 200 IU/mL. Therefore, several factors affect the detection of HBV DNA in plasma/serum (Table 5). Among these multiple factors, the sensitivity of the performed technique, specimen volume, the studied subjects and the status of collection of the analyzed blood specimens can be considered [90–95]. Occult hepatitis B infection (OBI) is distinguished by a negative HBsAg status, the detection of very low levels of HBV-DNA replication in the liver and the absence or presence of HBV DNA in the blood [95].
Table 5.
Clinical implications in occult hepatitis B infection (OBI) phase of HBV infection
| Occult hepatitis B infection (OBI) | |||||||
|---|---|---|---|---|---|---|---|
| Seropositive OBI | Seronegative OBI | ||||||
| HBV DNA in serum | HBsAg | Anti-HBc IgG | Anti-hepatitis B surface (HBs) IgG | HBV DNA in serum/liver tissue | HBsAg | Anti-HBc IgG | Anti-hepatitis B surface (HBs) IgG |
| Detectable | Negative | Positive | Positive/Negative | Detectable | Negative | Negative | Negative |
Although in the diagnosis of OBI, viral load < 200 IU/mL is determined for patients, nevertheless, in ˃ 90% of patients diagnosed with OBI, the level of viral load in the serum has been detected at approximately 20 IU/mL [96]. A study conducted in China reported significantly higher rates of maternal levels of viral loads greater than 100 IU/mL among OBI-positive babies compared to infants with negative results for OBI [97]. In adults, roughly 1–3% of OBI have been identified following HB vaccination [98, 99]. In a cohort study, among vaccinated subjects, the prevalence of OBI in anti-HBc-positive individuals 16/334 (4.8%) was greater than that of anti-HBc-negative individuals: 0/392 (0%) [100]. Another study on HB-vaccinated blood donors demonstrated three primary OBIs and 17 OBIs [101]. The prevalence of OBI in children is not fully understood, but it is potentially possible. In childhood and infancy, HBV infection is easily transmitted. Babies infected with HBV account for 25–30% of chronic carriers until the end of life. Therefore, progression to liver cirrhosis and liver cancer occurs at a higher rate in these children compared to being HBV-infected at an older age. Several possible factors, including high viral load levels in the mother, hyporesponse/nonresponse to the HB vaccine, decreased titers for anti-HBs protection and the development of mutations in the S region associated with vaccine escape, could play a role in OBI among HB-vaccinated children [97]. Several studies have reported the prevalence of OBI in HBV-vaccinated individuals in different populations (Table 6).
Table 6.
Prevalence of occult hepatitis B infection (OBI) among vaccinated individuals
| Studied population | N | OBI Number N (%) | Factors involved | Country | References |
|---|---|---|---|---|---|
| HBV vaccinated children | N = 46 | 5 (10.9) | C139S vaccine-escape mutant, Variation and deletion in pre-S region | Taiwan | [102] |
| Anti-HBs-positive young adults | N = 2919 | 124 (4.2) | Mutations at the “a” epitope/outside of the “a” epitope | China | [99] |
| Children with HBsAg-positive mothers | N = 75 | 21 [28] | 13 (62%): at least one mutation, 10: G145R mutations | Iran | [103] |
| Outpatients | N = 282 | 1 | Tanzania | [104] | |
| Newborns with HBsAg positive mothers | N = 100 | 2 [2] | African (French island in the Mozambican canal) | [105] | |
| School healthy children | N = 229 | Five cases | Amino acid mutation in S region/Pre-S region | Indonesia | [106] |
| Babies with HBsAg-positive mothers | N = 213 | 89 [42] | India | [107] | |
| Immunized children | N = 327 | 10 (3.1) | China | [108] | |
| HB vaccinated babies with HBsAg-positive mothers | N = 183 | 9 (4.92) |
Maternal viral loads > 100 IU/mL, Four pre-S/S sequences of C/D genotype: S143L escape mutation |
China | [97] |
Individuals at risk of HBV infection following HB vaccine Failure
A study demonstrates the importance of HB vaccination in healthcare workers (HCWs). Most HCWs are unvaccinated. Anti-HBs titers decrease gradually over time in HB vaccinated individuals. In a significant population of HCWs who have received full doses of vaccination, as well as partially vaccinated individuals, protective titers against HBV infection are inadequate. The study emphasizes the importance of universal screening for anti-HBs titer, HBs antigen measurement and administration of booster dose of HB vaccine in HCWs [75]. Given the ability of HBV to cause chronic and sub-clinical infection, the ability to be transmitted through body fluids and blood in non-immune individuals, as well as vaccination failures, especially in healthcare systems, transmission may occur inadvertently. Revaccination has been considered an appropriate solution in non-responders/low-responders in developed countries. Subsequently, seroconversion has been achieved in most low-responders. However, after revaccinations, approximately half of non-responders do not increase HBs antibody levels. A study reported that a patient developed acute hepatitis B infection despite receiving five doses of HB vaccine. The primary response to HB vaccination in this patient was moderate, with a high level of anti-HBs > 1000 IU/l being reported after booster HB vaccination (recombinant DNA vaccine). However, this patient showed HBV infection and developed acute hepatitis 14 years after this booster recombinant DNA vaccination. Their study described hepatitis B vaccination failure even after a high level of anti-HBs was raised following recombinant DNA hepatitis B vaccination [109].
Reducing hepatocellular carcinoma (HCC) rates following HB vaccination
One of the most important malignancies worldwide is liver cancer, which is known as the third leading cause of mortality rate among various cancers [110]. Hepatocellular carcinoma (HCC) is one of the most challenging and important public health problems. Approximately 90% of primary liver cancer cases are attributed to HCC. One of the obstacles to the effectiveness of treatment is the late diagnosis of HCC in the advanced phase of the disease [111]. The prevalence rate of HBV infection, as well as the incidence of HCC, has been significantly reduced following universal HB vaccination [112, 113]. Fortunately, effective HB vaccination can prevent HBV and the progression of the disease to HCC.
Improved response through hepatitis B third-generation vaccines
Recent studies have demonstrated that a tri-antigenic hepatitis B vaccine (S, pre-S1, and pre-S2 antigens) induced a more robust immune response compared to a mono-antigenic hepatitis B vaccine (S antigen) [114, 115] (Table 7).
The role of various host genetic factors
Recently, the critical role of genetic factors involved in the non-responders to the HBV vaccine has been identified. The most important factors include single nucleotide polymorphisms (SNPs) in toll-like receptors (TLRs), cytokine receptors/cytokine, chemokine, and human leukocyte antigen (HLA) haplotypes [119].
Single nucleotide polymorphisms (SNPs) in chemokine and cytokine genes
Single nucleotide polymorphisms (SNPs) occurring in the IL-4 and IL-2 loci associated with insertion/deletion (indels) variants in the IL12B gene are closely related to HBV vaccine response status [120]. Furthermore, the prominent role of chemokines and cytokines in HBV vaccine response has been identified. For example, one SNP (rs355687) in CXCL13 and three SNPs (rs3922, rs497916, rs676925) in CXCR5 have been detected, which have shown a close relationship with the HBV vaccine response [121]. Also, a significant relationship between SNPs and hepatitis B vaccine host response was reported. Interestingly, a potential genetic relation was identified between the major histocompatibility complex (MHC) locus located on the chromosome 6 (rs5000563) and hepatitis B vaccine response. On the other hand, based on possible predictions, other SNPs in this region are able to change the sequence of proteins [122].
Human leukocyte antigen (HLA)
Several studies have reported the relation of HB vaccine response with genetic variation identified at the HLA locus [120, 122–124]. A low rate of HLA-CW6 and a high rate of HLA-A24 and HLA-A11 have been demonstrated to be related to non-responders HB vaccine [125]. Non-response to HB vaccine has been reported in both healthy individuals and patients with celiac disease associated with DQ2, DR3 and HLA-B8 haplotypes [126]. It has been reported that although HLA-B13 is associated with adequate response to vaccine, however, HLA-DRB1*0401X0201, DRB1*11/13, DRB1*0401X and DRB1*04X haplotypes are associated with no-response [127]. Furthermore, the association of poor response to the HB vaccine with some HLA alleles, such as DRB1*08(-), DRB1*07 and B62, has been identified [128]. Associations between poor vaccine responses and DRB1*04, DRB1*07, DRB1 *03 (DRB1*0301), DRB1*1302 and DQB1*02 HLA alleles have also been identified [129]. Recently, one cohort study evaluates the relationship between response vaccine antigens and HLA in Bangladeshi children. Association of greater HBV antibody response with DPB1*04:01 was reported. Hepatitis B surface antigens (HBsAg) epitopes bind with higher affinity to DPB1*04:01 dimers. Most probably, the evolutionary pressure created in the “a” determinant region leads to the HBV vaccine-escape mutants [130]. Various main factors related to poor/low response to HBV vaccination are demonstrated in Table 8.
Table 8.
Various main factors associated with poor/low response to HBV vaccination
| Main factors | References |
|---|---|
| Inappropriate storage condition during transport of vaccine | [131] |
| Smoking | [55, 69, 132] |
| HIV infection | [133] |
| Obesity | [55, 69, 79, 132] |
| Diabetes | [76, 80, 134] |
| Insulin-resistance | [79] |
| HLA haplotypes | [130, 135] |
| Celiac disease | [136–140] |
| Gender (males) | [69, 77, 79] |
| Age | [55, 69, 73, 76, 77, 79, 132] |
| HCV | [141–143] |
| Hemodialysis patients | [144] |
| Peritoneal dialysis patients | [145] |
| Chronic kidney disease | [146] |
| Genetic factors | [147] |
| Body mass index (BMI) | [132] |
| Low birth weight | [71] |
| Immune cells | [148] |
| Immunosuppressive drugs | [76] |
Hepatitis B vaccine-escape mutants
HBV surface antigen (HBsAg) is known as the main envelope protein of the virus. On the other hand, HBsAg contains the main epitopes as well as the important regions involved in the binding of HBV to hepatocytes, which neutralizing antibodies recognize the HBsAg [149]. Protection against HBV infection and immunity undergo changes following mutations in the “a” determinant. These changes can induce HBV vaccine evasion [150]. In 1988, these mutations’ vital impact and importance were reported through babies with HBsAg-positive mothers for the first time. In these infants, infection was identified despite administering the HBV vaccine and HBIG. This mutation causes a change in “a” determinant. As a result, it is impossible to identify the virus through neutralizing antibodies produced after vaccination, and following this significant alteration, infection occurs [150]. The mechanism of mutation in the S gene is not fully understood; however, some hypotheses have suggested the occurrence of these spontaneous mutations in the S gene due to the interference of the host’s immune system [151, 152]. In Taiwan, out of 12 children who received the HBV vaccine, 8 were identified as detectable HBV DNA-positive in serum. One of them demonstrated “a” determinant mutation [153]. However, other HBV vaccine-escape mutations such as M133L, P120S/E, T116N, Q129H/R, I/T126A/N/I/S, D144A/E, D144A/H, P142S, G145R/A, K141E, A128V, G130N and M133L/T have been identified to be associated with the “a” determinant. Still, despite widespread vaccination, the G145R as predominant circulating escape mutant has been reported [149, 154–157]. One case report study demonstrated that an infant developed an occult HBV infection (OBI) after a liver-transplantation despite receiving the HBV vaccine. Specific vaccine-escape mutations have been shown to play a critical role in the transmission of OBI [158]. A case report study has shown that despite receiving the vaccine, a person progressed to an acute hepatitis B infection. Q129H vaccine-escape mutation in the “a” determinant with the mechanism of altering HBsAg antigenicity, and also critical and effective change in the attachment of anti-HBs to HBsAg, has been involved in vaccine evasion [159]. Recently, HBV-specific vaccine-escape mutations have been reported in Bangladesh [160]. In addition, in the Netherlands, HBV vaccine-escape mutations in the HBsAg due to genetic changes have been identified [161]. These mutations have the ability to prevent the recognition of HBsAg by antibodies and can also control the secretion of HBsAg [162]. Vaccine-escape mutations related to hepatitis B surface antigen “a” determinant region/major hydrophilic region are demonstrated in Table 9.
Table 9.
Vaccine-escape mutations associated with hepatitis B surface antigen “a” determinant region/major hydrophilic region
| Amino acid substitutions/Position of codon | References |
|---|---|
| G145R/A | [103, 150, 153, 157, 158, 161, 163–176] |
| T116N | [177] |
| M133L | [178, 179] |
| P120S/E | [167, 168, 180] |
| D144A/E | [161, 169, 179, 180] |
| I/T126A/N/I/S | [169, 180] |
| K141E | [166] |
| Q129H/R | [159, 161, 178, 179] |
| P142S | [169, 170] |
| N146S | [153] |
| T131I | [171] |
| Ile/Thr-126-Asn/Ser | [173] |
| I126S/N | [157] |
| T126A | [157] |
| 128 V | [160] |
| Y100C | [161] |
| L109I | [161] |
| T118R | [161] |
| P120T/S | [161] |
| T126I/S/A | [161] |
| P127T/L | [161] |
| T131S/I | [161] |
| M133I | [161] |
| F/Y134N/L | [161] |
| T140I | [161] |
| S143L | [161] |
| A168V | [161] |
| T126I | [158] |
| P120T | [158] |
| P142S | [158] |
| M133I | [176] |
Suggested solutions
In non-responder individuals who do not develop adequate seroprotection after three doses of conventional HB vaccination, 1 to 3 additional doses of HB vaccine are usually recommended [42]. In high-risk individuals with a history of HB vaccination and reduced titers (anti-HBs < 10 mIU/ml), a booster dose is recommended for a protective effect [181]. Receiving a fourth dose of HB vaccine has been reported as an efficient strategy in HCV-infected individuals [142]. Although some studies have reported a poor response to HBV vaccination despite treatment, especially in patients with chronic HCV [182, 183], HBV revaccination after treatment of HCV infection is recommended for non-responders [184]. On the other hand, despite receiving a booster dose of HBV vaccine, celiac patients are still considered to be poor responders to the vaccine [140]. Therefore, according to the controversial findings, it seems that achieving a hepatitis B vaccine with long‐term protection is still challenging. In addition, after receiving the 3-antigenic hepatitis B vaccine, more robust and faster seroprotection is induced, which can provide earlier seroprotection than the mono-antigenic vaccine. The results obtained from recent studies highlight the clinical importance of using third-generation vaccines, especially in non-responders and susceptible high-risk individuals at risk of HBV infection [114, 115]. Also, some studies have new suggestions. One case report study demonstrated that protection was induced by intradermal HBV vaccination in HBV vaccine non-responder HIV-infected patients [185]. Among general persons and hemodialysis who were non-responders to intramuscular HB vaccination, intradermal HB vaccination has produced an efficient response [186]. Recently, immunogenicity and safety of therapeutic HBV vaccine have been demonstrated in both chronic HBV patients and healthy individuals [187]. According to the best of our knowledge, in addition to receiving booster doses, the hepatitis B third-generation vaccines and new adjuvanted recombinant HB vaccines are targeted and highly effective alternatives to conventional HB vaccines, and universal use of these new comprehensive approaches is recommended.
Conclusion
Despite the occurrence of HB vaccine-escape mutations and the decrease in HBsAb titer over time, the best and most important strategy against this blood-borne virus is still universal vaccination. In contrast, depending on the development of HBsAg-specific mutations and, as a result, the impairment of HBsAg recognition by neutralizing antibodies and the genetic factors of the host involved in the response to the vaccine, the efficiency of HBV vaccination is affected. Therefore, despite the highly efficient HBV vaccine, producing a vaccine with long-term protection in all targeted Individuals is still very challenging. In addition, monitoring and following the HBsAb titer continuously is an appropriate solution for high-risk individuals and healthcare workers, especially in endemic regions. If the HBsAb level decreases in these individuals, it is suggested that a booster dose be prescribed if needed. The HB vaccination strategy remains valuable despite viral and host factors involved in HB vaccine failure. Fortunately, not only receiving booster doses in high-risk groups is an appropriate approach, but also the use of new adjuvant vaccines and third-generation HB vaccines is recommended as the best alternatives to conventional HB vaccines, particularly in no/poor responders. However, this issue should be followed quite seriously.
Authors’ contributions
A.M. and M.F.A. and s.M.J. wrote the main manuscript text. A.M. and A.Kh. prepared Figs. 1–4. A.M. and M.N.T. and S.H.A. prepared Tables 1–9. All authors reviewed the manuscript.
Funding
No funding.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interests
The authors declare no competing interests.
Ethical approval and consent to participate
All information and data used in this review are extracted from published articles. So, data are not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
- 2.Sheena BS, Hiebert L, Han H, Ippolito H, Abbasi-Kangevari M, Abbasi-Kangevari Z, Abbastabar H, Abdoli A, Ali HA, Adane MM, Adegboye OA. Global, regional, and national burden of hepatitis B, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Gastroenterol Hepatol. 2022;7(9):796–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu YC, Huang DQ, Nguyen MH. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat Rev Gastroenterol Hepatol. 2023;20(8):524–37. [DOI] [PubMed] [Google Scholar]
- 4.Shen C, Jiang X, Li M, Luo Y. Hepatitis virus and hepatocellular carcinoma: recent advances. Cancers. 2023;15(2):533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao H, Zhou X, Zhou YH. Hepatitis B vaccine development and implementation. Hum Vaccin Immunother. 2020;16(7):1533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumberg BS, Alter HJ, Visnich S. A “New” antigen in Leukemia sera. JAMA. 1965;191:541–6. [DOI] [PubMed] [Google Scholar]
- 7.Dane DS, Cameron CH, Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970;1(7649):695–8. [DOI] [PubMed] [Google Scholar]
- 8.Krugman S, Giles JP, Hammond J. Hepatitis virus: effect of heat on the infectivity and antigenicity of the MS-1 and MS-2 strains. J Infect Dis. 1970;122(5):432–6. [DOI] [PubMed] [Google Scholar]
- 9.Krugman S, Giles JP, Hammond J. Viral hepatitis, type B (MS-2 strain). Studies on active immunization. Jama. 1971;217(1):41–5. [PubMed] [Google Scholar]
- 10.Maynard JE, Berquist KR, Krushak DH, Purcell RH. Experimental infection of chimpanzees with the virus of hepatitis B. Nature. 1972;237(5357):514–5. [DOI] [PubMed] [Google Scholar]
- 11.Buynak EB, Roehm RR, Tytell AA, Bertland AU 2nd, Lampson GP, Hilleman MR. Development and chimpanzee testing of a vaccine against human hepatitis B. Proc Soc Exp Biol Med Soc Exp Biol Med (New York, NY). 1976;151(4):694–700. [DOI] [PubMed] [Google Scholar]
- 12.Szmuness W, Stevens CE, Harley EJ, Zang EA, Oleszko WR, William DC, et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980;303(15):833–41. [DOI] [PubMed] [Google Scholar]
- 13.Francis DP, Feorino PM, McDougal S, Warfield D, Getchell J, Cabradilla C, et al. The safety of the hepatitis B vaccine. Inactivation of the AIDS virus during routine vaccine manufacture. Jama. 1986;256(7):869–72. [PubMed] [Google Scholar]
- 14.Maupas P, Goudeau A, Coursaget P, Drucker J, Bagros P. Immunisation against hepatitis B in man. Lancet. 1976;1(7974):1367–70. [DOI] [PubMed] [Google Scholar]
- 15.Pattyn J, Hendrickx G, Vorsters A, Van Damme P. Hepatitis B Vaccines. J Infect Dis. 2021;224(12 Suppl 2):S343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens CE, Szmuness W, Goodman AI, Weseley SA, Fotino M. Hepatitis B vaccine: immune responses in haemodialysis patients. Lancet. 1980;2(8206):1211–3. [DOI] [PubMed] [Google Scholar]
- 17.Maupas P, Chiron JP, Barin F, Coursaget P, Goudeau A, Perrin J, et al. Efficacy of hepatitis B vaccine in prevention of early HBsAg carrier state in children controlled trial in an endemic area (Senegal). Lancet. 1981;1(8215):289–92. [DOI] [PubMed] [Google Scholar]
- 18.Krugman S. The newly licensed hepatitis B vaccine. Characteristics and indications for use. Jama. 1982;247(14):2012–5. [PubMed] [Google Scholar]
- 19.Goudeau A, Dubois F, Barin F, Dubois MC, Coursaget P. Hepatitis B vaccine: clinical trials in high-risk settings in France (September 1975-September 1982). Dev Biol Stand. 1983;54:267–84. [PubMed] [Google Scholar]
- 20.Szmuness W, Stevens CE, Harley EJ, Zang EA, Alter HJ, Taylor PE, et al. Hepatitis B vaccine in medical staff of hemodialysis units: efficacy and subtype cross-protection. N Engl J Med. 1982;307(24):1481–6. [DOI] [PubMed] [Google Scholar]
- 21.Hilleman MR, McAleer WJ, Buynak EB, McLean AA. The preparation and safety of hepatitis B vaccine. J Infect. 1983;7(Suppl 1):3–8. [DOI] [PubMed] [Google Scholar]
- 22.McAleer WJ, Buynak EB, Maigetter RZ, Wampler DE, Miller WJ, Hilleman MR. Human hepatitis B vaccine from recombinant yeast. Nature. 1984;307(5947):178–80. [DOI] [PubMed] [Google Scholar]
- 23.Emini EA, Ellis RW, Miller WJ, McAleer WJ, Scolnick EM, Gerety RJ. Production and immunological analysis of recombinant hepatitis B vaccine. J Infect. 1986;13(Suppl 1):3–9. [DOI] [PubMed] [Google Scholar]
- 24.Hilleman MR. Yeast recombinant hepatitis B vaccine. Infection. 1987;15(1):3–7. [DOI] [PubMed] [Google Scholar]
- 25.Valenzuela P, Medina A, Rutter WJ, Ammerer G, Hall BD. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature. 1982;298(5872):347–50. [DOI] [PubMed] [Google Scholar]
- 26.Romano L, Zanetti AR. Hepatitis B vaccination: a historical overview with a focus on the Italian achievements. Viruses. 2022;14(7):1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shouval D. Hepatitis B vaccines. J Hepatol. 2003;39(Suppl 1):S70–6. [DOI] [PubMed] [Google Scholar]
- 28.Yum JS, Ahn BC, Jo HJ, Kim DY, Kim KH, Kim HS, et al. Use of pre-S protein-containing hepatitis B virus surface antigens and a powerful adjuvant to develop an immune therapy for chronic hepatitis B virus infection. Clin Vaccine Immunol: CVI. 2012;19(2):120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shouval D, Ilan Y, Adler R, Deepen R, Panet A, Even-Chen Z, et al. Improved immunogenicity in mice of a mammalian cell-derived recombinant hepatitis B vaccine containing pre-S1 and pre-S2 antigens as compared with conventional yeast-derived vaccines. Vaccine. 1994;12(15):1453–9. [DOI] [PubMed] [Google Scholar]
- 30.Zuckerman JN, Zuckerman AJ, Symington I, Du W, Williams A, Dickson B, et al. Evaluation of a new hepatitis B triple-antigen vaccine in inadequate responders to current vaccines. Hepatology. 2001;34(4 Pt 1):798–802. [DOI] [PubMed] [Google Scholar]
- 31.Rendi-Wagner P, Shouval D, Genton B, Lurie Y, Rümke H, Boland G, et al. Comparative immunogenicity of a PreS/S hepatitis B vaccine in non- and low responders to conventional vaccine. Vaccine. 2006;24(15):2781–9. [DOI] [PubMed] [Google Scholar]
- 32.Lo CM, Lau GK, Chan SC, Fan ST, Wong J. Efficacy of a pre-S containing vaccine in patients receiving lamivudine prophylaxis after liver transplantation for chronic hepatitis B. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg. 2007;7(2):434–9. [DOI] [PubMed] [Google Scholar]
- 33.Tong NK, Beran J, Kee SA, Miguel JL, Sánchez C, Bayas JM, et al. Immunogenicity and safety of an adjuvanted hepatitis B vaccine in pre-hemodialysis and hemodialysis patients. Kidney Int. 2005;68(5):2298–303. [DOI] [PubMed] [Google Scholar]
- 34.Beran J. Safety and immunogenicity of a new hepatitis B vaccine for the protection of patients with renal insufficiency including pre-haemodialysis and haemodialysis patients. Expert Opin Biol Ther. 2008;8(2):235–47. [DOI] [PubMed] [Google Scholar]
- 35.Jacques P, Moens G, Desombere I, Dewijngaert J, Leroux-Roels G, Wettendorff M, et al. The immunogenicity and reactogenicity profile of a candidate hepatitis B vaccine in an adult vaccine non-responder population. Vaccine. 2002;20(31–32):3644–9. [DOI] [PubMed] [Google Scholar]
- 36.María FP, María BA, Darío RO, Paula AP, Vicent LJ, Inés FP, et al. Immunogenicity of the hepatitis B vaccine adjuvanted with AS04C in patients with biological therapies. Vaccine. 2023;41(3):744–9. [DOI] [PubMed] [Google Scholar]
- 37.Jackson S, Lentino J, Kopp J, Murray L, Ellison W, Rhee M, et al. Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine. 2018;36(5):668–74. [DOI] [PubMed] [Google Scholar]
- 38.Marks KM, Kang M, Umbleja T, Avihingsanon A, Sugandhavesa P, Cox AL, et al. Immunogenicity and safety of hepatitis B virus (HBV) vaccine with a toll-like receptor 9 agonist adjuvant in HBV vaccine-naïve people with human immunodeficiency virus. Clin Infect Dis. 2023;77(3):414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vilajeliu A, Sequera VG, García-Basteiro AL, Sicuri E, Aldea M, Velasco C, et al. Immunogenicity and immunization costs of adjuvanted versus non-adjuvanted hepatitis B vaccine in chronic kidney disease patients. Hum Vaccin Immunother. 2016;12(9):2317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Girndt M, Plüer M, Dellanna F, Michelsen AK, Beige J, Toussaint K, et al. Immunogenicity and safety of a booster dose of the hepatitis B vaccine HepB-CpG (HEPLISAV-B®) compared with HepB-Eng (Engerix-B®) and HepB-AS04 (Fendrix®) in adults receiving hemodialysis who previously received hepatitis B vaccination and are not seroprotected: results of a randomized, multicenter phase 3 study. Hum Vaccin Immunother. 2022;18(6):2136912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee GH, Lim SG. CpG-Adjuvanted hepatitis B vaccine (HEPLISAV-B®) update. Expert Rev Vaccines. 2021;20(5):487–95. [DOI] [PubMed] [Google Scholar]
- 42.Halperin SA, Ward BJ, Dionne M, Langley JM, McNeil SA, Smith B, et al. Immunogenicity of an investigational hepatitis B vaccine (hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide) in nonresponders to licensed hepatitis B vaccine. Hum Vaccin Immunother. 2013;9(7):1438–44. [DOI] [PubMed] [Google Scholar]
- 43.Shan P, Wang Z, Li J, Wei D, Zhang Z, Hao S, et al. A new nano adjuvant of PF3 used for an enhanced hepatitis B vaccine. Front Bioeng Biotechnol. 2022;10: 903424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koc ÖM, de Smedt P, Kremer C, Robaeys G, van Damme P, Hens N, et al. Immunogenicity and safety of HBAI20 Hepatitis B vaccine in non-responders: double-blinded, randomised, controlled phase 2 trial. Liver Int. 2021;41(10):2318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koc ÖM, Savelkoul PHM, van Loo IHM, Peeters A, Oude Lashof AML. Safety and immunogenicity of HBAI20 Hepatitis B vaccine in healthy naïve and nonresponding adults. J Viral Hepatitis. 2018;25(9):1048–56. [DOI] [PubMed] [Google Scholar]
- 46.Fabrizi F, Cerutti R, Garcia-Agudo R, Bellincioni C, Porata G, Frontini G, et al. Adjuvanted recombinant HBV vaccine (HBV-AS04) is effective over extended follow-up in dialysis population. An open-label non randomized trial. Clin Res Hepatol Gastroenterol. 2020;44(6):905–12. [DOI] [PubMed] [Google Scholar]
- 47.Beran J, Hobzova L, Wertzova V, Kuriyakose S, Leyssen M, Surquin M, et al. Safety and immunogenicity of an investigational adjuvanted hepatitis B vaccine (HB-AS02V) in healthy adults. Hum Vaccin. 2010;6(7):578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernández Sánchez-Escalonilla S, Gonzalez-Rubio J, Najera A, Cantero Escribano JM, Molina Cabrero FJ, García GJ. Using the AS04C-adjuvanted hepatitis B vaccine in patients classified as non-responders. Trans R Soc Trop Med Hyg. 2024;118(3):170–7. [DOI] [PubMed] [Google Scholar]
- 49.Reilly-Evans B, Dudzik B, Costlow DJ, Hartmann C, Khalsa AM, Kassis C, et al. Observational study evaluating the seroprotection of HepB-alum vaccine and HepB-CpG vaccine in people with HIV. Open Forum Infect Dis. 2023;10(6):ofad267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beasley RP, Hwang LY, Lee GC, Lan CC, Roan CH, Huang FY, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2(8359):1099–102. [DOI] [PubMed] [Google Scholar]
- 51.Lo KJ, Tsai YT, Lee SD, Wu TC, Wang JY, Chen GH, et al. Immunoprophylaxis of infection with hepatitis B virus in infants born to hepatitis B surface antigen-positive carrier mothers. J Infect Dis. 1985;152(4):817–22. [DOI] [PubMed] [Google Scholar]
- 52.Hsu HM, Chen DS, Chuang CH, Lu JC, Jwo DM, Lee CC, et al. Efficacy of a mass hepatitis B vaccination program in Taiwan. Studies on 3464 infants of hepatitis B surface antigen-carrier mothers. Jama. 1988;260(15):2231–5. [PubMed] [Google Scholar]
- 53.Chang KC, Chang MH, Chen HL, Wu JF, Chang CH, Hsu HY, et al. Universal infant hepatitis B virus (HBV) vaccination for 35 years: moving toward the eradication of HBV. J Infect Dis. 2022;225(3):431–5. [DOI] [PubMed] [Google Scholar]
- 54.Zhang W, Xu C, Rui Y, Chen J, Chen T, Dai Y, et al. Efficacy of the hepatitis B vaccine alone in the prevention of hepatitis B perinatal transmission in infants born to hepatitis B e antigen-negative carrier mothers. J Virus Erad. 2022;8(2): 100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meier MA, Berger CT. A simple clinical score to identify likely hepatitis B vaccination non-responders-data from a retrospective single center study. BMC Infect Dis. 2020;20(1):891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Filippelli M, Lionetti E, Gennaro A, Lanzafame A, Arrigo T, Salpietro C, et al. Hepatitis B vaccine by intradermal route in non responder patients: an update. World J Gastroenterol. 2014;20(30):10383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen DS. Hepatitis B vaccination: the key towards elimination and eradication of hepatitis B. J Hepatol. 2009;50(4):805–16. [DOI] [PubMed] [Google Scholar]
- 58.Yanny B, Konyn P, Najarian LM, Mitry A, Saab S. Management approaches to hepatitis B virus vaccination nonresponse. Gastroenterol Hepatol. 2019;15(2):93–9. [PMC free article] [PubMed] [Google Scholar]
- 59.Saco TV, Strauss AT, Ledford DK. Hepatitis B vaccine nonresponders: possible mechanisms and solutions. Ann Allergy, Asthma Immunol. 2018;121(3):320–7. [DOI] [PubMed] [Google Scholar]
- 60.Tao I, Compaoré TR, Diarra B, Djigma F, Zohoncon TM, Assih M, et al. Seroepidemiology of hepatitis B and C viruses in the general population of burkina faso. Hepat Res Treat. 2014;2014: 781843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marion SA, Tomm Pastore M, Pi DW, Mathias RG. Long-term follow-up of hepatitis B vaccine in infants of carrier mothers. Am J Epidemiol. 1994;140(8):734–46. [DOI] [PubMed] [Google Scholar]
- 62.Kramvis A, Mammas IN, Spandidos DA. Exploring the optimal vaccination strategy against hepatitis B virus in childhood (Review). Biomed Rep. 2023;19(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adugna A, Demeke G, Toru M, Tsehay D, Esmael A, Mihret A, et al. Reduced protective efficacy of hepatitis B vaccine among fully vaccinated children in Ethiopia. PLoS ONE. 2023;18(7): e0288355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sood A, Singh D, Mehta S, Midha V, Kumar R. Response to hepatitis B vaccine in preterm babies. Indian J Gastroenterol. 2002;21(2):52–4. [PubMed] [Google Scholar]
- 65.Freitas da Motta MS, Mussi-Pinhata MM, Jorge SM, Tachibana Yoshida CF, Sandoval de Souza CB. Immunogenicity of hepatitis B vaccine in preterm and full term infants vaccinated within the first week of life. Vaccine. 2002;20(11–12):1557–62. [DOI] [PubMed] [Google Scholar]
- 66.Blondheim O, Bader D, Abend M, Peniakov M, Reich D, Potesman I, et al. Immunogenicity of hepatitis B vaccine in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998;79(3):F206–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cabrales J, Idrees N, Dgayli K, Dokmak A, Jaber B, Balakrishnan V. Predictors of immune response to hepatitis B vaccine and clinical outcomes in dialysis patients. Infect Dis Trop Med. 2023;9:e1174. [Google Scholar]
- 68.Youssef DM, El-Shal AS, Elbehidy RM, Fouda MA, Shalaby SM, El Hawy LL, et al. Hepatitis B immunization status in children with chronic kidney disease: experience at a single center, Egypt. J Clin Med. 2023;12(5):1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. Immunogenicity of hepatitis B Vaccines. Implications for persons at occupational risk of hepatitis B virus infection. Am J Prev Med. 1998;15(1):1–8. [DOI] [PubMed] [Google Scholar]
- 70.Coppola N, Corvino AR, De Pascalis S, Signoriello G, Di Fiore E, Nienhaus A, et al. The long-term immunogenicity of recombinant hepatitis B virus (HBV) vaccine: contribution of universal HBV vaccination in Italy. BMC Infect Dis. 2015;15:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han K, Shao X, Zheng H, Wu C, Zhu J, Zheng X, et al. Revaccination of non- and low- responders after a standard three dose hepatitis B vaccine schedule. Hum Vaccin Immunother. 2012;8(12):1845–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hassan S, Ziba F. Antibody titer in Iranian children 6 years after hepatitis B vaccine administration. Vaccine. 2007;25(17):3511–4. [DOI] [PubMed] [Google Scholar]
- 73.Lall M, Sen S, Patrikar S, Karade S, Gupta RM. Post vaccination antibody titres of hepatitis B surface antigen (anti-HBs) in a mixed cohort of health care workers. Med J, Armed Forces India. 2022;78(2):198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sahana HV, Sarala N, Prasad SR. Decrease in Anti-HBs antibodies over time in medical students and healthcare workers after hepatitis B vaccination. Biomed Res Int. 2017;2017:1327492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Batra V, Goswami A, Dadhich S, Kothari D, Bhargava N. Hepatitis B immunization in healthcare workers. Ann Gastroenterol. 2015;28(2):276–80. [PMC free article] [PubMed] [Google Scholar]
- 76.Nashibi R, Alavi SM, Yousefi F, Salmanzadeh S, Moogahi S, Ahmadi F, et al. Post-vaccination immunity against hepatitis B virus and predictors for non-responders among medical staff. Jundishapur J Microbiol. 2015;8(3): e19579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeeshan M, Jabeen K, Ali AN, Ali AW, Farooqui SZ, Mehraj V, et al. Evaluation of immune response to Hepatitis B vaccine in health care workers at a tertiary care hospital in Pakistan: an observational prospective study. BMC Infect Dis. 2007;7:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zamani F, Fallahian F, Hashemi F, Shamsaei Z, Alavian SM. Immune response to hepatitis B vaccine in health-care workers. Saudi J Kidney Dis Transplant. 2011;22(1):179–84. [PubMed] [Google Scholar]
- 79.Tkachenko LI, Maleev VV, Putrenok LS. Evaluation of seroconversion after vaccination of medical staff against HBV infection. Zh Mikrobiol Epidemiol Immunobiol. 2013;5:69–74. [PubMed] [Google Scholar]
- 80.Leonardi S, Vitaliti G, Garozzo MT, Miraglia del Giudice M, Marseglia G, La Rosa M. Hepatitis B vaccination failure in children with diabetes mellitus? The debate continues. Hum Vaccines Immunother. 2012;8(4):448–52. [DOI] [PubMed] [Google Scholar]
- 81.Shamsizadeh A, Makvandi M, Shoushtari GA. Prevalence of anti hepatitis B surface antibody among children in Ahvaz, Iran, five years after vaccination. Jundishapur J Microbiol. 2011;4(2):49–54. [Google Scholar]
- 82.Hung WL, Wu JF, Ni YH, Chen HL, Chiang CL, Chang MH, et al. Occult hepatitis B virus and surface antigen mutant infection in healthy vaccinated cohorts and children with various forms of hepatitis and multiple transfusions. Liver Int. 2019;39(6):1052–61. [DOI] [PubMed] [Google Scholar]
- 83.Tang Y, Liu X, Lu X, He Q, Li G, Zou Y. Occult hepatitis B virus infection in maintenance hemodialysis patients: prevalence and mutations in “a” determinant. Int J Med Sci. 2020;17(15):2299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bubonja-Šonje M, Peruč D, Abram M, Mohar-Vitezić B. Prevalence of occult hepatitis B virus infection and characterisation of hepatitis B surface antigen mutants among adults in western Croatia. Ann Hepatol. 2024;29(1): 101156. [DOI] [PubMed] [Google Scholar]
- 85.Wu CC, Chen YS, Cao L, Chen XW, Lu MJ. Hepatitis B virus infection: Defective surface antigen expression and pathogenesis. World J Gastroenterol. 2018;24(31):3488–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. J Hepatol. 2007;46(1):160–70. [DOI] [PubMed] [Google Scholar]
- 87.Tabor E, Hoofnagle JH, Smallwood LA, Drucker JA, Pineda-Tamondong GC, Ni LY, et al. Studies of donors who transmit posttransfusion hepatitis. Transfusion. 1979;19(6):725–31. [DOI] [PubMed] [Google Scholar]
- 88.Grob P, Jilg W, Bornhak H, Gerken G, Gerlich W, Günther S, et al. Serological pattern “anti-HBc alone”: report on a workshop. J Med Virol. 2000;62(4):450–5. [DOI] [PubMed] [Google Scholar]
- 89.Hu KQ. Occult hepatitis B virus infection and its clinical implications. J Viral Hepatitis. 2002;9(4):243–57. [DOI] [PubMed] [Google Scholar]
- 90.Kazemi-Shirazi L, Petermann D, Müller C. Hepatitis B virus DNA in sera and liver tissue of HBsAg negative patients with chronic hepatitis C. J Hepatol. 2000;33(5):785–90. [DOI] [PubMed] [Google Scholar]
- 91.Kannangai R, Vivekanandan P, Netski D, Mehta S, Kirk GD, Thomas DL, et al. Liver enzyme flares and occult hepatitis B in persons with chronic hepatitis C infection. J Clin Virol. 2007;39(2):101–5. [DOI] [PubMed] [Google Scholar]
- 92.Chemin I, Guillaud O, Queyron PC, Trépo C. Close monitoring of serum HBV DNA levels and liver enzymes levels is most useful in the management of patients with occult HBV infection. J Hepatol. 2009;51(4):824–5. [DOI] [PubMed] [Google Scholar]
- 93.Saitta C, Musolino C, Marabello G, Martino D, Leonardi MS, Pollicino T, et al. Risk of occult hepatitis B virus infection reactivation in patients with solid tumours undergoing chemotherapy. Dig Liver Dis. 2013;45(8):683–6. [DOI] [PubMed] [Google Scholar]
- 94.Candotti D, Assennato SM, Laperche S, Allain JP, Levicnik-Stezinar S. Multiple HBV transfusion transmissions from undetected occult infections: revising the minimal infectious dose. Gut. 2019;68(2):313–21. [DOI] [PubMed] [Google Scholar]
- 95.Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71(2):397–408. [DOI] [PubMed] [Google Scholar]
- 96.Yuen MF, Lee CK, Wong DK, Fung J, Hung I, Hsu A, et al. Prevalence of occult hepatitis B infection in a highly endemic area for chronic hepatitis B: a study of a large blood donor population. Gut. 2010;59(10):1389–93. [DOI] [PubMed] [Google Scholar]
- 97.Su H, Zhang Y, Xu D, Wang B, Zhang L, Li D, et al. Occult hepatitis B virus infection in anti-HBs-positive infants born to HBsAg-positive mothers in China. PLoS ONE. 2013;8(8): e70768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen SJ, Zhao YX, Fang Y, Xu WZ, Ma YX, Song ZW, et al. Viral deletions among healthy young Chinese adults with occult hepatitis B virus infection. Virus Res. 2012;163(1):197–201. [DOI] [PubMed] [Google Scholar]
- 99.Xu L, Wei Y, Chen T, Lu J, Zhu CL, Ni Z, et al. Occult HBV infection in anti-HBs-positive young adults after neonatal HB vaccination. Vaccine. 2010;28(37):5986–92. [DOI] [PubMed] [Google Scholar]
- 100.Hsu HY, Chang MH, Ni YH, Chiang CL, Wu JF, Chen HL. Universal infant immunization and occult hepatitis B virus infection in children and adolescents: a population-based study. Hepatology. 2015;61(4):1183–91. [DOI] [PubMed] [Google Scholar]
- 101.Zheng X, Ye X, Du P, Zeng J, Zhu W, Yang B, et al. High prevalence of anti-hepatitis B core antigen in hepatitis B virus-vaccinated Chinese blood donors suggests insufficient protection but little threat to the blood supply. Transfusion. 2015;55(4):890–7. [DOI] [PubMed] [Google Scholar]
- 102.Mu SC, Lin YM, Jow GM, Chen BF. Occult hepatitis B virus infection in hepatitis B vaccinated children in Taiwan. J Hepatol. 2009;50(2):264–72. [DOI] [PubMed] [Google Scholar]
- 103.Shahmoradi S, Yahyapour Y, Mahmoodi M, Alavian SM, Fazeli Z, Jazayeri SM. High prevalence of occult hepatitis B virus infection in children born to HBsAg-positive mothers despite prophylaxis with hepatitis B vaccination and HBIG. J Hepatol. 2012;57(3):515–21. [DOI] [PubMed] [Google Scholar]
- 104.Meschi S, Schepisi MS, Nicastri E, Bevilacqua N, Castilletti C, Sciarrone MR, et al. The prevalence of antibodies to human herpesvirus 8 and hepatitis B virus in patients in two hospitals in Tanzania. J Med Virol. 2010;82(9):1569–75. [DOI] [PubMed] [Google Scholar]
- 105.Chakvetadze C, Roussin C, Roux J, Mallet V, Petinelli ME, Pol S. Efficacy of hepatitis B sero-vaccination in newborns of African HBsAg positive mothers. Vaccine. 2011;29(16):2846–9. [DOI] [PubMed] [Google Scholar]
- 106.Utsumi T, Yano Y, Lusida MI, Amin M, Soetjipto HH, et al. Serologic and molecular characteristics of hepatitis B virus among school children in East Java, Indonesia. Am J Trop Med Hyg. 2010;83(1):189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pande C, Sarin SK, Patra S, Kumar A, Mishra S, Srivastava S, et al. Hepatitis B vaccination with or without hepatitis B immunoglobulin at birth to babies born of HBsAg-positive mothers prevents overt HBV transmission but may not prevent occult HBV infection in babies: a randomized controlled trial. J Viral Hepatitis. 2013;20(11):801–10. [DOI] [PubMed] [Google Scholar]
- 108.Zhuge S, Ge C, Yang Y, Cui Y, Yue X, Zhang Z, et al. The prevalence of occult HBV infection in immunized children with HBsAg-positive parents: a hospital-based analysis. Hep Intl. 2020;14(4):503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boot HJ, van der Waaij LA, Schirm J, Kallenberg CG, van Steenbergen J, Wolters B. Acute hepatitis B in a healthcare worker: a case report of genuine vaccination failure. J Hepatol. 2009;50(2):426–31. [DOI] [PubMed] [Google Scholar]
- 110.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 111.Rizzo GEM, Cabibbo G, Craxì A. Hepatitis B virus-associated hepatocellular carcinoma. Viruses. 2022;14(5):986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wong GL, Hui VW, Yip TC, Liang LY, Zhang X, Tse YK, et al. Universal HBV vaccination dramatically reduces the prevalence of HBV infection and incidence of hepatocellular carcinoma. Aliment Pharmacol Ther. 2022;56(5):869–77. [DOI] [PubMed] [Google Scholar]
- 113.Dasgupta P, Henshaw C, Youlden DR, Clark PJ, Aitken JF, Baade PD. Global trends in incidence rates of primary adult liver cancers: a systematic review and meta-analysis. Front Oncol. 2020;10:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vesikari T, Langley JM, Segall N, Ward BJ, Cooper C, Poliquin G, et al. Immunogenicity and safety of a tri-antigenic versus a mono-antigenic hepatitis B vaccine in adults (PROTECT): a randomised, double-blind, phase 3 trial. Lancet Infect Dis. 2021;21(9):1271–81. [DOI] [PubMed] [Google Scholar]
- 115.Vesikari T, Finn A, van Damme P, Leroux-Roels I, Leroux-Roels G, Segall N, et al. Immunogenicity and safety of a 3-Antigen hepatitis B vaccine vs a single-antigen hepatitis B vaccine: a phase 3 randomized clinical trial. JAMA Netw Open. 2021;4(10): e2128652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Esaulenko EV, Yakovlev AA, Volkov GA, Sukhoruk AA, Surkov KG, Kruglyakov PV, et al. Efficacy and safety of a 3-Antigen (Pre-S1/Pre-S2/S) hepatitis B vaccine: results of a phase 3 randomized clinical trial in the Russian federation. Clin Infect Dis. 2021;73(9):e3333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Atsmon J, Machluf N, Yayon-Gur V, Sabbah C, Spaans JN, Yassin-Rajkumar B, et al. Rapid and high seroprotection rates achieved with a tri-antigenic Hepatitis B vaccine in healthy young adults: results from a phase IV study. Vaccine. 2021;39(8):1328–32. [DOI] [PubMed] [Google Scholar]
- 118.Alon D, Stein GY, Hadas-Golan V, Tau L, Brosh T, Turner D. Immunogenicity of Sci-B-Vac (a third-generation hepatitis B vaccine) in HIV-positive adults. Isr Med Assoc J: IMAJ. 2017;19(3):143–6. [PubMed] [Google Scholar]
- 119.Chen J, Liang Z, Lu F, Fang X, Liu S, Zeng Y, et al. Toll-like receptors and cytokines/cytokine receptors polymorphisms associate with non-response to hepatitis B vaccine. Vaccine. 2011;29(4):706–11. [DOI] [PubMed] [Google Scholar]
- 120.Wang C, Tang J, Song W, Lobashevsky E, Wilson CM, Kaslow RA. HLA and cytokine gene polymorphisms are independently associated with responses to hepatitis B vaccination. Hepatology. 2004;39(4):978–88. [DOI] [PubMed] [Google Scholar]
- 121.Duan Z, Chen X, Liang Z, Zeng Y, Zhu F, Long L, et al. Genetic polymorphisms of CXCR5 and CXCL13 are associated with non-responsiveness to the hepatitis B vaccine. Vaccine. 2014;32(41):5316–22. [DOI] [PubMed] [Google Scholar]
- 122.Davila S, Froeling FE, Tan A, Bonnard C, Boland GJ, Snippe H, et al. New genetic associations detected in a host response study to hepatitis B vaccine. Genes Immun. 2010;11(3):232–8. [DOI] [PubMed] [Google Scholar]
- 123.Höhler T, Meyer CU, Notghi A, Stradmann-Bellinghausen B, Schneider PM, Starke R, et al. The influence of major histocompatibility complex class II genes and T-cell Vbeta repertoire on response to immunization with HBsAg. Hum Immunol. 1998;59(4):212–8. [DOI] [PubMed] [Google Scholar]
- 124.Hsu HY, Chang MH, Ho HN, Hsieh RP, Lee SD, Chen DS, et al. Association of HLA-DR14-DR52 with low responsiveness to hepatitis B vaccine in Chinese residents in Taiwan. Vaccine. 1993;11(14):1437–40. [DOI] [PubMed] [Google Scholar]
- 125.Albayrak A, Ertek M, Tasyaran MA, Pirim I. Role of HLA allele polymorphism in chronic hepatitis B virus infection and HBV vaccine sensitivity in patients from eastern Turkey. Biochem Genet. 2011;49(3–4):258–69. [DOI] [PubMed] [Google Scholar]
- 126.Vitaliti G, Praticò AD, Cimino C, Di Dio G, Lionetti E, La Rosa M, et al. Hepatitis B vaccine in celiac disease: yesterday, today and tomorrow. World J Gastroenterol. 2013;19(6):838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mert G, Sengul A, Gul HC, Karakas A, Eyigun CP. The role of human leukocyte antigen tissue groups in hepatitis B virus vaccination in Turkey. J Microbiol, Immunol, Infect. 2014;47(1):9–14. [DOI] [PubMed] [Google Scholar]
- 128.Yoon JH, Shin S, In J, Chang JY, Song EY, Roh EY. Association of HLA alleles with the responsiveness to hepatitis B virus vaccination in Korean infants. Vaccine. 2014;32(43):5638–44. [DOI] [PubMed] [Google Scholar]
- 129.Li ZK, Nie JJ, Li J, Zhuang H. The effect of HLA on immunological response to hepatitis B vaccine in healthy people: a meta-analysis. Vaccine. 2013;31(40):4355–61. [DOI] [PubMed] [Google Scholar]
- 130.Butler-Laporte G, Auckland K, Noor Z, Kabir M, Alam M, Carstensen T, et al. Targeting hepatitis B vaccine escape using immunogenetics in Bangladeshi infants. MedRxiv. 2023;90:1576. [Google Scholar]
- 131.Edstam JS, Dulmaa N, Tsendjav O, Dambasuren B, Densmaa B. Exposure of hepatitis B vaccine to freezing temperatures during transport to rural health centers in Mongolia. Prev Med. 2004;39(2):384–8. [DOI] [PubMed] [Google Scholar]
- 132.Ingardia CJ, Kelley L, Steinfeld JD, Wax JR. Hepatitis B vaccination in pregnancy: factors influencing efficacy. Obstet Gynecol. 1999;93(6):983–6. [DOI] [PubMed] [Google Scholar]
- 133.Haban H, Benchekroun S, Sadeq M, Benjouad A, Amzazi S, Oumzil H, et al. Assessment of the HBV vaccine response in a group of HIV-infected children in Morocco. BMC Public Health. 2017;17(1):752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pozzilli P, Arduini P, Visalli N, Sutherland J, Pezzella M, Galli C, et al. Reduced protection against hepatitis B virus following vaccination in patients with type 1 (insulin-dependent) diabetes. Diabetologia. 1987;30(10):817–9. [DOI] [PubMed] [Google Scholar]
- 135.Desombere I, Willems A, Leroux-Roels G. Response to hepatitis B vaccine: multiple HLA genes are involved. Tissue Antigens. 1998;51(6):593–604. [DOI] [PubMed] [Google Scholar]
- 136.Filippelli M, Garozzo MT, Capizzi A, Spina M, Manti S, Tardino L, et al. Immune response to hepatitis B virus vaccine in celiac subjects at diagnosis. World J Hepatol. 2016;8(26):1105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Leonardi S, Spina M, Spicuzza L, Rotolo N, La Rosa M. Hepatitis B vaccination failure in celiac disease: is there a need to reassess current immunization strategies? Vaccine. 2009;27(43):6030–3. [DOI] [PubMed] [Google Scholar]
- 138.Park SD, Markowitz J, Pettei M, Weinstein T, Sison CP, Swiss SR, et al. Failure to respond to hepatitis B vaccine in children with celiac disease. J Pediatr Gastroenterol Nutr. 2007;44(4):431–5. [DOI] [PubMed] [Google Scholar]
- 139.Ahishali E, Boztas G, Akyuz F, Ibrisim D, Poturoglu S, Pinarbasi B, et al. Response to hepatitis B vaccination in patients with celiac disease. Dig Dis Sci. 2008;53(8):2156–9. [DOI] [PubMed] [Google Scholar]
- 140.Zingone F, Morisco F, Zanetti A, Romanò L, Portella G, Capone P, et al. Long-term antibody persistence and immune memory to hepatitis B virus in adult celiac patients vaccinated as adolescents. Vaccine. 2011;29(5):1005–8. [DOI] [PubMed] [Google Scholar]
- 141.Ashhab AA, Rodin H, Campos M, Abu-Sulb A, Hall JA, Powell J, et al. Response to hepatitis B virus vaccination in individuals with chronic hepatitis C virus infection. PLoS ONE. 2020;15(8): e0237398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Medeiros RP, Terrault NA, Mazo DF, Oliveira CP, Dodge J, Zitelli PM, et al. Impaired anti-HBV vaccine response in non-cirrhotic chronic HCV is not overcome by double dose regimen: randomized control trial. Ann Hepatol. 2023;28(2): 100891. [DOI] [PubMed] [Google Scholar]
- 143.Moorman JP, Zhang CL, Ni L, Ma CJ, Zhang Y, Wu XY, et al. Impaired hepatitis B vaccine responses during chronic hepatitis C infection: involvement of the PD-1 pathway in regulating CD4(+) T cell responses. Vaccine. 2011;29(17):3169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Eleftheriadis T, Liakopoulos V, Antoniadi G, Stefanidis I, Galaktidou G. Indoleamine 2,3-dioxygenase is increased in hemodialysis patients and affects immune response to hepatitis B vaccination. Vaccine. 2011;29(12):2242–7. [DOI] [PubMed] [Google Scholar]
- 145.Lin SY, Liu JH, Lin CC, Wang SM, Tsai CA, Chou CY, et al. Comparison of hepatitis B surface antibody decay rates after vaccination between hemodialysis and peritoneal dialysis patients. Vaccine. 2011;29(21):3738–41. [DOI] [PubMed] [Google Scholar]
- 146.Zitt E, Sprenger-Mähr H, Knoll F, Neyer U, Lhotta K. Vitamin D deficiency is associated with poor response to active hepatitis B immunisation in patients with chronic kidney disease. Vaccine. 2012;30(5):931–5. [DOI] [PubMed] [Google Scholar]
- 147.Yan K, Cai W, Cao F, Sun H, Chen S, Xu R, et al. Genetic effects have a dominant role on poor responses to infant vaccination to hepatitis B virus. J Hum Genet. 2013;58(5):293–7. [DOI] [PubMed] [Google Scholar]
- 148.Körber N, Pohl L, Weinberger B, Grubeck-Loebenstein B, Wawer A, Knolle PA, et al. Hepatitis B vaccine non-responders show higher frequencies of CD24(high)CD38(high) regulatory B cells and lower levels of IL-10 expression compared to responders. Front Immunol. 2021;12: 713351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kay A, Zoulim F. Hepatitis B virus genetic variability and evolution. Virus Res. 2007;127(2):164–76. [DOI] [PubMed] [Google Scholar]
- 150.Zanetti AR, Tanzi E, Manzillo G, Maio G, Sbreglia C, Caporaso N, et al. Hepatitis B variant in Europe. Lancet. 1988;2(8620):1132–3. [DOI] [PubMed] [Google Scholar]
- 151.Lada O, Benhamou Y, Poynard T, Thibault V. Coexistence of hepatitis B surface antigen (HBs Ag) and anti-HBs antibodies in chronic hepatitis B virus carriers: influence of “a” determinant variants. J Virol. 2006;80(6):2968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mesenas SJ, Chow WC, Zhao Y, Lim GK, Oon CJ, Ng HS. Wild-type and ‘a’ epitope variants in chronic hepatitis B virus carriers positive for hepatitis B surface antigen and antibody. J Gastroenterol Hepatol. 2002;17(2):148–52. [DOI] [PubMed] [Google Scholar]
- 153.Hsu HY, Chang MH, Liaw SH, Ni YH, Chen HL. Changes of hepatitis B surface antigen variants in carrier children before and after universal vaccination in Taiwan. Hepatology. 1999;30(5):1312–7. [DOI] [PubMed] [Google Scholar]
- 154.Echevarría JM, Avellón A. Hepatitis B virus genetic diversity. J Med Virol. 2006;78(Suppl 1):S36-42. [DOI] [PubMed] [Google Scholar]
- 155.Lazarevic I. Clinical implications of hepatitis B virus mutations: recent advances. World J Gastroenterol. 2014;20(24):7653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cooreman MP, Leroux-Roels G, Paulij WP. Vaccine- and hepatitis B immune globulin-induced escape mutations of hepatitis B virus surface antigen. J Biomed Sci. 2001;8(3):237–47. [DOI] [PubMed] [Google Scholar]
- 157.Yan B, Lv J, Feng Y, Liu J, Ji F, Xu A, et al. Temporal trend of hepatitis B surface mutations in the post-immunization period: 9 years of surveillance (2005–2013) in eastern China. Sci Rep. 2017;7(1):6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Salpini R, Pietrobattista A, Piermatteo L, Basso MS, Bellocchi MC, Liccardo D, et al. Establishment of a seronegative occult infection with an active hepatitis B virus reservoir enriched of vaccine escape mutations in a vaccinated infant after liver transplantation. J Infect Dis. 2019;220(12):1935–9. [DOI] [PubMed] [Google Scholar]
- 159.Luongo M, Critelli R, Grottola A, Gitto S, Bernabucci V, Bevini M, et al. Acute hepatitis B caused by a vaccine-escape HBV strain in vaccinated subject: sequence analysis and therapeutic strategy. J Clin Virol. 2015;62:89–91. [DOI] [PubMed] [Google Scholar]
- 160.Hossain MG, Ueda K. A meta-analysis on genetic variability of RT/HBsAg overlapping region of hepatitis B virus (HBV) isolates of Bangladesh. Infect Agents Cancer. 2019;14:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cremer J, Hofstraat SHI, van Heiningen F, Veldhuijzen IK, van Benthem BHB, Benschop KSM. Genetic variation of hepatitis B surface antigen among acute and chronic hepatitis B virus infections in The Netherlands. J Med Virol. 2018;90(10):1576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Piermatteo L, D’Anna S, Bertoli A, Bellocchi M, Carioti L, Fabeni L, et al. Unexpected rise in the circulation of complex HBV variants enriched of HBsAg vaccine-escape mutations in HBV genotype-D: potential impact on HBsAg detection/quantification and vaccination strategies. Emerg Microbes Infect. 2023;12(1):2219347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Carman WF, Zanetti AR, Karayiannis P, Waters J, Manzillo G, Tanzi E, et al. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990;336(8711):325–9. [DOI] [PubMed] [Google Scholar]
- 164.Zuckerman JN, Zuckerman AJ. Mutations of the surface protein of hepatitis B virus. Antiviral Res. 2003;60(2):75–8. [DOI] [PubMed] [Google Scholar]
- 165.Fujii H, Moriyama K, Sakamoto N, Kondo T, Yasuda K, Hiraizumi Y, et al. Gly145 to Arg substitution in HBs antigen of immune escape mutant of hepatitis B virus. Biochem Biophys Res Commun. 1992;184(3):1152–7. [DOI] [PubMed] [Google Scholar]
- 166.Coleman PF, Chen YC, Mushahwar IK. Immunoassay detection of hepatitis B surface antigen mutants. J Med Virol. 1999;59(1):19–24. [DOI] [PubMed] [Google Scholar]
- 167.Ireland JH, O’Donnell B, Basuni AA, Kean JD, Wallace LA, Lau GK, et al. Reactivity of 13 in vitro expressed hepatitis B surface antigen variants in 7 commercial diagnostic assays. Hepatology. 2000;31(5):1176–82. [DOI] [PubMed] [Google Scholar]
- 168.Lee KM, Kim YS, Ko YY, Yoo BM, Lee KJ, Kim JH, et al. Emergence of vaccine-induced escape mutant of hepatitis B virus with multiple surface gene mutations in a Korean child. J Korean Med Sci. 2001;16(3):359–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Moerman B, Moons V, Sommer H, Schmitt Y, Stetter M. Evaluation of sensitivity for wild type and mutant forms of hepatitis B surface antigen by four commercial HBsAg assays. Clin Lab. 2004;50(3–4):159–62. [PubMed] [Google Scholar]
- 170.Seddigh-Tonekaboni S, Lim WL, Young B, Hou JL, Waters J, Luo KX, et al. Hepatitis B surface antigen variants in vaccinees, blood donors and an interferon-treated patient. J Viral Hepatitis. 2001;8(2):154–8. [DOI] [PubMed] [Google Scholar]
- 171.Hsu HY, Chang MH, Ni YH, Chen HL. Survey of hepatitis B surface variant infection in children 15 years after a nationwide vaccination programme in Taiwan. Gut. 2004;53(10):1499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Komatsu H, Inui A, Umetsu S, Tsunoda T, Sogo T, Konishi Y, et al. Evaluation of the G145R Mutant of the Hepatitis B Virus as a Minor Strain in Mother-to-Child Transmission. PLoS ONE. 2016;11(11): e0165674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.He C, Nomura F, Itoga S, Isobe K, Nakai T. Prevalence of vaccine-induced escape mutants of hepatitis B virus in the adult population in China: a prospective study in 176 restaurant employees. J Gastroenterol Hepatol. 2001;16(12):1373–7. [DOI] [PubMed] [Google Scholar]
- 174.Okamoto H, Yano K, Nozaki Y, Matsui A, Miyazaki H, Yamamoto K, et al. Mutations within the S gene of hepatitis B virus transmitted from mothers to babies immunized with hepatitis B immune globulin and vaccine. Pediatr Res. 1992;32(3):264–8. [DOI] [PubMed] [Google Scholar]
- 175.Chakravarty R, Neogi M, Roychowdhury S, Panda CK. Presence of hepatitis B surface antigen mutant G145R DNA in the peripheral blood leukocytes of the family members of an asymptomatic carrier and evidence of its horizontal transmission. Virus Res. 2002;90(1–2):133–41. [DOI] [PubMed] [Google Scholar]
- 176.Latthaphasavang V, Vanhems P, Ngo-Giang-Huong N, Sibounlang P, Paboriboune P, Malato L, et al. Perinatal hepatitis B virus transmission in Lao PDR: a prospective cohort study. PLoS ONE. 2019;14(4): e0215011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chong-Jin O, Wei Ning C, Shiuan K, Gek KL. Identification of hepatitis B surface antigen variants with alterations outside the “a” determinant in immunized Singapore infants. J Infect Dis. 1999;179(1):259–63. [DOI] [PubMed] [Google Scholar]
- 178.Ho M, Mau Y, Lu C, Huang S, Hsu L, Lin S, et al. Patterns of circulating hepatitis B surface antigen variants among vaccinated children born to hepatitis B surface antigen carrier and non-carrier mothers. A population-based comparative study. J Biomed Sci. 1998;5(5):355–62. [DOI] [PubMed] [Google Scholar]
- 179.Oon CJ, Tan KL, Harrison T, Zuckerman A. Natural history of hepatitis B surface antigen mutants in children. Lancet. 1996;348(9040):1524. [DOI] [PubMed] [Google Scholar]
- 180.Ngui SL, O’Connell S, Eglin RP, Heptonstall J, Teo CG. Low detection rate and maternal provenance of hepatitis B virus S gene mutants in cases of failed postnatal immunoprophylaxis in England and Wales. J Infect Dis. 1997;176(5):1360–5. [DOI] [PubMed] [Google Scholar]
- 181.Posuwan N, Vorayingyong A, Jaroonvanichkul V, Wasitthankasem R, Wanlapakorn N, Vongpunsawad S, et al. Implementation of hepatitis B vaccine in high-risk young adults with waning immunity. PLoS ONE. 2018;13(8): e0202637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Abd El-Wahab EW, Metwally M, Lotfy N. Effectiveness of hepatitis B vaccination in chronic HCV patients after successful generic direct acting antiviral therapy: significance of isolated hepatitis B core antibodies. Trop Med Int Health. 2021;26(8):882–94. [DOI] [PubMed] [Google Scholar]
- 183.Em S, Nour El Din D, Elawady MA, Ragab SY, El-Eraky TEJBMJ. Response to hepatitis B vaccine in chronic hepatitis c patients treated with direct acting antivirals. Benha Med J. 2022;39(2):346–56. [Google Scholar]
- 184.Powell JG, Goble SR, Debes JD. Revaccination for hepatitis B in previous nonresponders following hepatitis C eradication. J Infect Dis. 2024;229(2):341–5. [DOI] [PubMed] [Google Scholar]
- 185.Das M, Vanar V, Martin DK, Walayat S, Patel J, Badshah MB, et al. Seroconverting nonresponder of high-dose intramuscular HBV vaccine with intradermal HBV vaccine: a case report. Medicine. 2017;96(46): e8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hanif FM, Mehmood N, Majid Z, Luck NH, Laeeq SM, Tasneem AA, et al. Successful response of intradermal hepatitis B vaccine in nonresponders of intramuscular hepatitis B vaccine in general and hemodialysis population. Saudi J Gastroenterol. 2020;26(6):306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cargill T, Cicconi P, Brown A, Holland L, Karanth B, Rutkowski K, et al. HBV001: phase I study evaluating the safety and immunogenicity of the therapeutic vaccine ChAdOx1-HBV. JHEP Rep. 2023;5(11): 100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.