Abstract
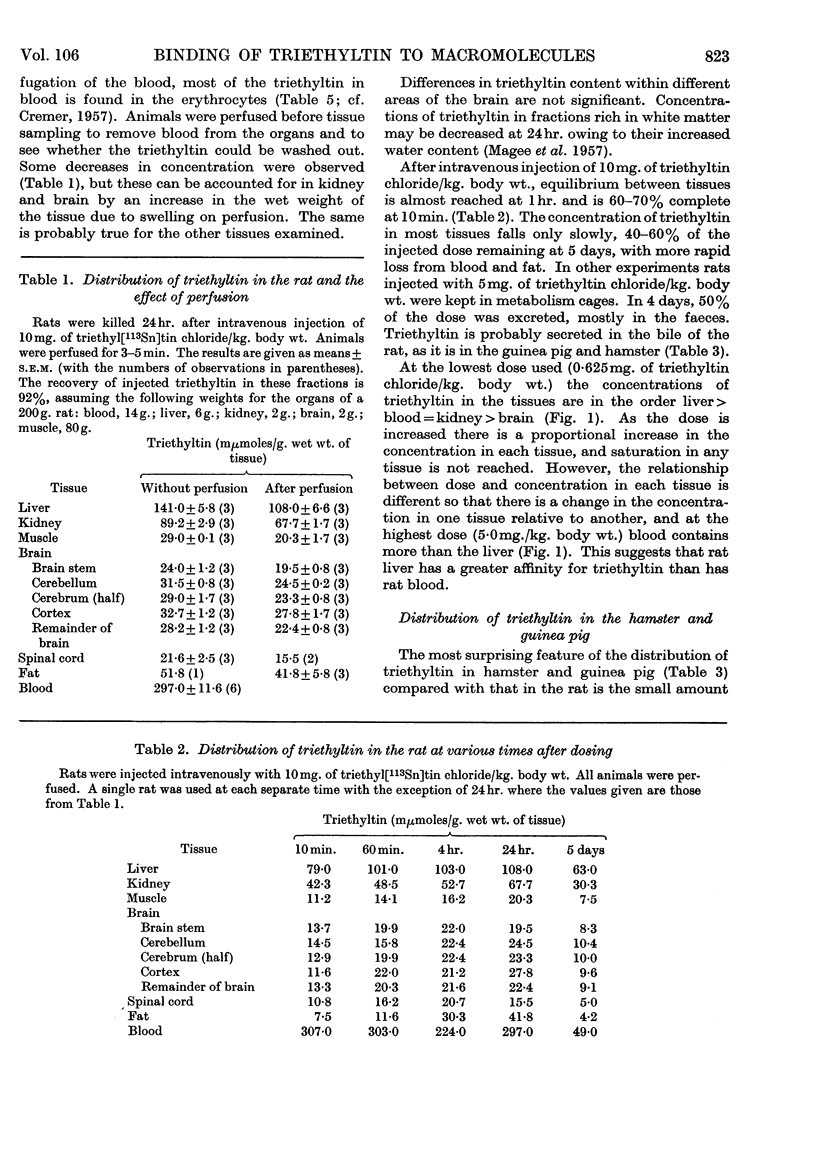
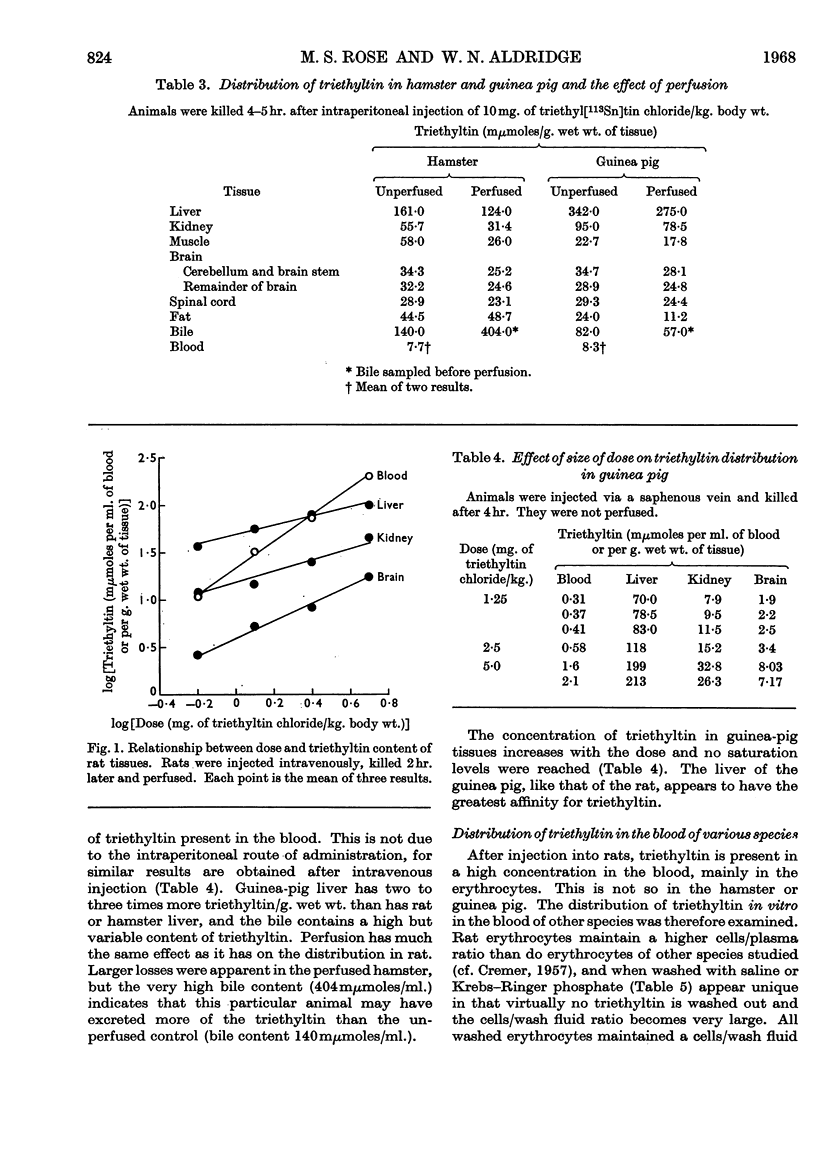
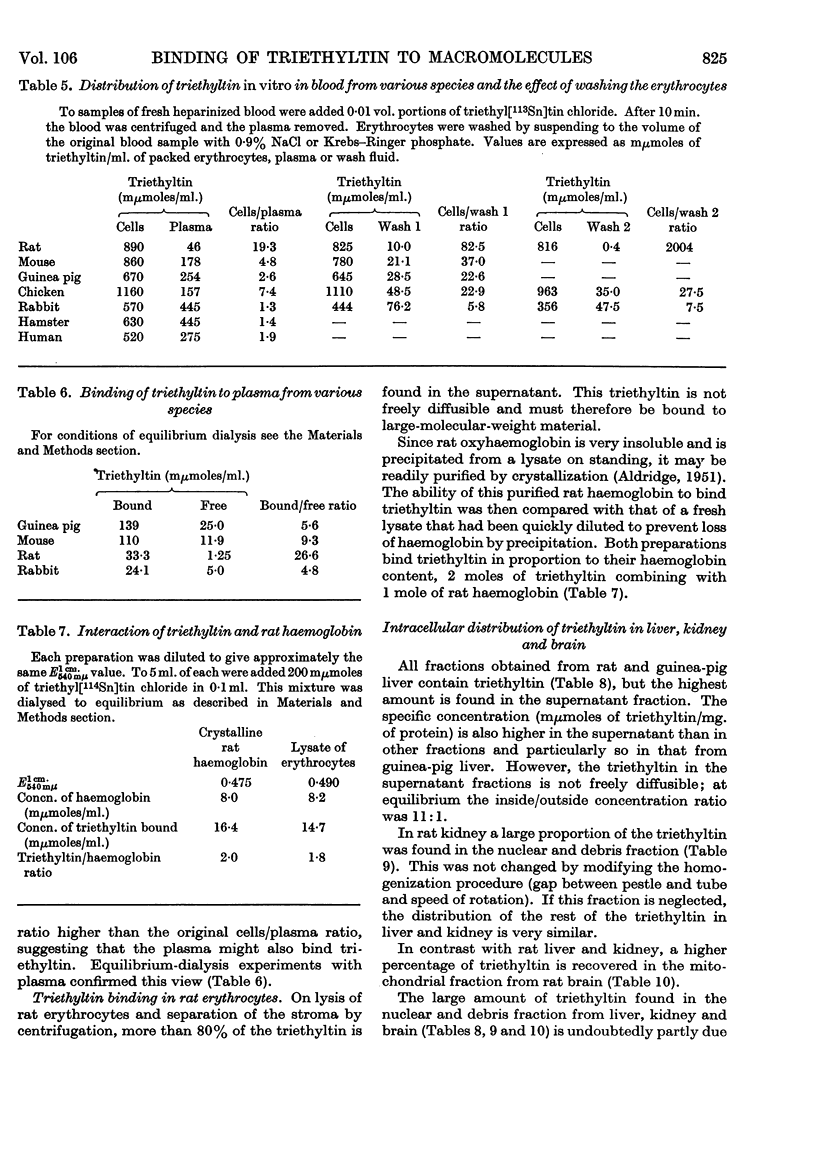
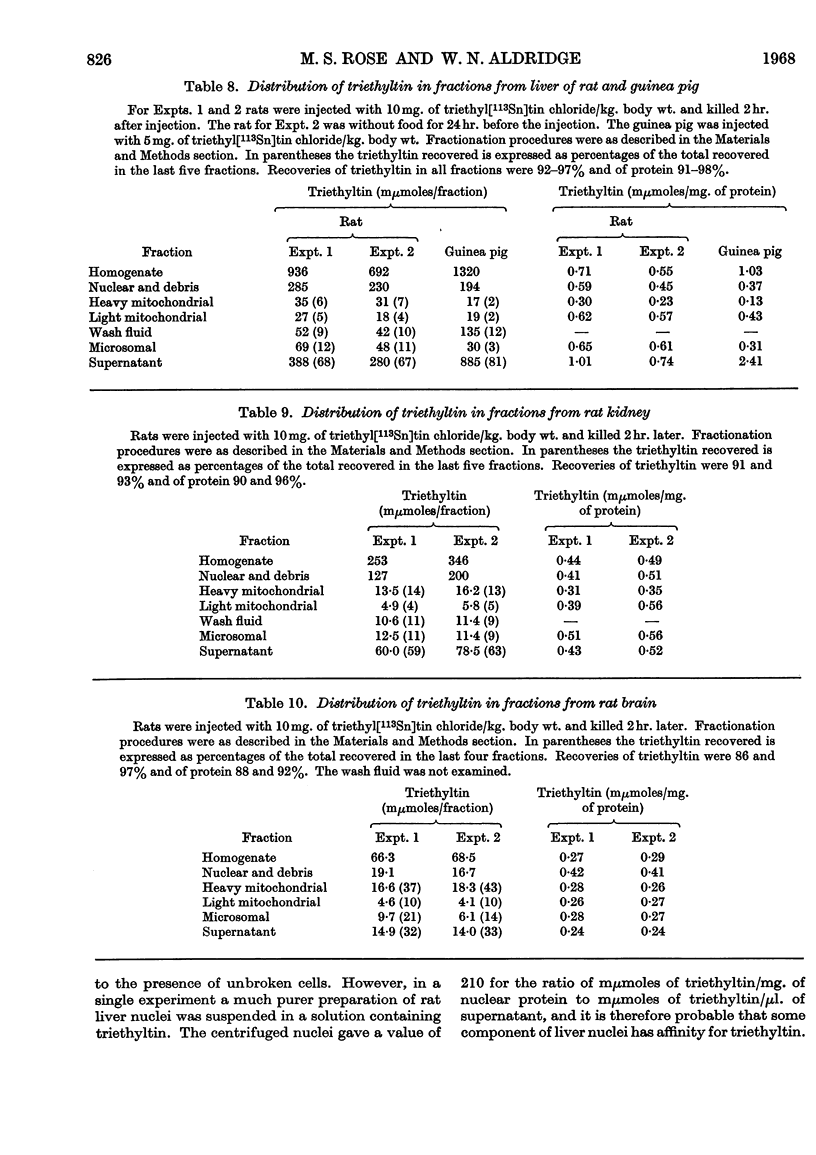
1. The distribution of triethyl[113Sn]tin chloride in the rat, guinea pig and hamster is not uniform, the highest concentrations being in rat blood and the liver of all three species. 2. Subcellular fractionation of rat liver, brain and kidney shows that triethyltin binds to all fractions to different extents. In the liver of the rat and guinea pig the supernatant fraction contains the largest amount and the highest specific concentration; this triethyltin is bound to a non-diffusible component. 3. Rat haemoglobin is responsible for the binding of triethyltin in rat blood (2 moles of triethyltin/mole of haemoglobin). Haemoglobins from other species have much less affinity for triethyltin. 4. A variety of other proteins do not bind triethyltin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. Adenosine triphosphatase in the microsomal fraction from rat brain. Biochem J. 1962 Jun;83:527–533. doi: 10.1042/bj0830527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDRIDGE W. N., CREMER J. E. The biochemistry of organo-tin compounds; diethyltin dichloride and triethyltin sulphate. Biochem J. 1955 Nov;61(3):406–418. doi: 10.1042/bj0610406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDRIDGE W. N., EMERY R. C., STREET B. W. A tissue homogenizer. Biochem J. 1960 Nov;77:326–327. doi: 10.1042/bj0770326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDRIDGE W. N., JOHNSON M. K. Cholinesterase, succinic dehydrogenase, nucleic acids, esterase and glutathione reductase in sub-cellular fractions from rat brain. Biochem J. 1959 Oct;73:270–276. doi: 10.1042/bj0730270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDRIDGE W. N. Liver and brain mitochondria. Biochem J. 1957 Nov;67(3):423–431. doi: 10.1042/bj0670423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDRIDGE W. N., THRELFALL C. J. Trialkyltins and oxidative phosphorylation. The [32P]phosphate-adenosine triphosphate-exchange reaction. Biochem J. 1961 May;79:214–219. doi: 10.1042/bj0790214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDRIDGE W. N. The biochemistry of organotin compounds: trialkyltins and oxidative phosphorylation. Biochem J. 1958 Jul;69(3):367–376. doi: 10.1042/bj0690367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDRIDGE W. N. The conversion of cyanogen chloride to cyanide in the presence of blood proteins and sulphydryl compounds. Biochem J. 1951 Mar;48(3):271–276. doi: 10.1042/bj0480271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge W. N., Street B. W. Oxidative phosphorylation. Biochemical effects and properties of trialkyltins. Biochem J. 1964 May;91(2):287–297. doi: 10.1042/bj0910287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODY T. M., MOORE K. E. Biochemical aspects of triethyltin toxicity. Fed Proc. 1962 Nov-Dec;21:1103–1106. [PubMed] [Google Scholar]
- CREMER J. E. A comparison of the action of triethyltin with other durgs on creatine phosphate levels in rat brain and diaphragm preparations. Biochem Pharmacol. 1961 May;6:153–160. doi: 10.1016/0006-2952(61)90159-9. [DOI] [PubMed] [Google Scholar]
- CREMER J. E. The biochemistry of organotin compounds; the conversion of tetraethyltin into triethyltin in mammals. Biochem J. 1958 Apr;68(4):685–692. doi: 10.1042/bj0680685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREMER J. E. The metabolism in vitro of tissue slices from rats given triethyltin compounds. Biochem J. 1957 Sep;67(1):87–96. doi: 10.1042/bj0670087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZMAN R., ALEU F., WILSON C. FURTHER OBSERVATIONS ON TRIETHYLTIN EDEMA. Arch Neurol. 1963 Aug;9:178–187. doi: 10.1001/archneur.1963.00460080088011. [DOI] [PubMed] [Google Scholar]
- MOORE K. E., BRODY T. M. The effect of triethyltin on oxidative phosphorylation and mitochondrial adenosine triphosphatase activation. Biochem Pharmacol. 1961 May;6:125–133. doi: 10.1016/0006-2952(61)90156-3. [DOI] [PubMed] [Google Scholar]
- Rose M. S., Aldridge W. N. Triethyltin and the incorporation of [32P] phosphate into rat brain phospholipids. J Neurochem. 1966 Feb;13(2):103–108. doi: 10.1111/j.1471-4159.1966.tb03337.x. [DOI] [PubMed] [Google Scholar]
- STONER H. B., BARNES J. M., DUFF J. I. Studies on the toxicity of alkyl tin compounds. Br J Pharmacol Chemother. 1955 Mar;10(1):16–25. doi: 10.1111/j.1476-5381.1955.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STONER H. B., THRELFALL C. J. The biochemistry of organotin compounds: effect of triethyltin sulphate on tissue phosphates in the rat. Biochem J. 1958 Jul;69(3):376–385. doi: 10.1042/bj0690376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STREICHER E. The thiocyanate space of rat brain in experimental cerebral edema. J Neuropathol Exp Neurol. 1962 Jul;21:437–441. doi: 10.1097/00005072-196207000-00011. [DOI] [PubMed] [Google Scholar]
- WHITTAKER V. P. The isolation and characterization of acetylcholine-containing particles from brain. Biochem J. 1959 Aug;72:694–706. doi: 10.1042/bj0720694. [DOI] [PMC free article] [PubMed] [Google Scholar]


