ABSTRACT
Background and Aim
Currently, available evidence suggests prostate‐specific antigen (PSA) is highly sensitive but poorly specific for prostate cancer detection in symptomatic patients and those with lower urinary tract symptoms. Serum ferritin and hepcidin, which are implicated in the pathogenesis of prostate cancers, may complement the diagnostic value of PSA. This study explored the correlation of serum PSA with ferritin and hepcidin as new and complementary diagnostic biomarkers for prostate disease.
Methods
This hospital‐based case–control study was conducted at Methodist Hospital, Wenchi, with 90 participants. Venous blood sample was taken for complete blood count, PSA, ferritin, and hepcidin measurements using Mindray automated hematology analyzer and ELISA, respectively. Data were analyzed with SPSS version 27, and p < 0.05 was considered statistically significant.
Results
Serum PSA levels were significantly higher among the Prostate Cancer patients compared to BPE patients [32.1 (18.2–47.6) vs. 18.3 (12.2–0.6), p < 0.001]. Levels of serum ferritin and hepcidin were found to be significantly higher in PCa patients compared to BPE and controls (p < 0.001). Serum PSA of prostate disease patients showed a strong positive correlation with levels of ferritin (r = 0.739, p < 0.001) but moderately correlated with serum hepcidin levels (r = 0.670, p < 0.001). Serum ferritin was found to be an excellent diagnostic marker for prostate cancer (AUC = 0.972, p < 0.001) and BPE (AUC = 0.900, p < 0.001). Serum hepcidin was a better marker for PCa (AUC = 0.911) but a poor BPE (AUC = 0.664, p = 0.023). Significant reduction in the levels of Hb, RBC, MCHC, and HCT but higher counts of TWBC and RDW‐CV were observed in patients with prostate cancers compared to those with BPE and the normal control group (p < 0.05).
Conclusion
High levels of serum ferritin and hepcidin significantly correlated directly with increased serum total PSA levels and could play a valuable role to complement the noninvasive total PSA to improve diagnostic accuracy.
Keywords: benign prostatic enlargement, ferritin, hepcidin, prostate cancer, prostate‐specific antigen
1. Introduction
Prostate cancer is the second most common cancer diagnosis in men and the fifth leading cause of death worldwide [1]. More than 80% of all prostate cancers occur in men above the age of 65, even though some cases of the disease have been reported in lower age groups. As of 2022, there was an estimated 1.47 million new cases and 396,792 deaths from prostate cancer worldwide [2]. Ghana has reported a high prevalence of 6%–10% in men aged 50 years and above [3].
Prostate‐specific antigen (PSA) has been widely employed as a clinical diagnostic biomarker for prostate cancer [4]. However, elevated PSA levels have also been noted in patients with benign prostatic enlargement (BPE), prostatitis, and after physical trauma to the prostate (such as a bicycle injury, a digital rectal exam, catheterization, etc.) [5].
This suggests that PSA is not always specific for prostate cancer. Additionally, PSA levels in people with prostate cancer might fall below the “normal” range of 4 ng/mL, which can lead to a misdiagnosis. Consequently, given its general lack of specificity and sensitivity, PSA may not be the best biomarker for prostate cancer [6].
Ferritin is the primary iron storage protein and plays a number of important roles in many physiological and pathological processes. An increasing number of studies have shown that circulating ferritin levels are elevated in patients with a variety of malignant types [7, 8, 9]. Ferritin may serve as a suitable biomarker in the diagnosis and prognosis of various cancer types, including prostate cancer [8, 10]. Prostate cancer induces hepcidin production, which could lead to iron deficiency anemia, increase cancer cell proliferation, and reduce apoptosis of cancer cells [11, 12]. This is typically viewed as an indirect result of the elevated cytokine levels found in cancer patients and their stimulatory effect on the production of hepatic hepcidin. The circulating ferritin levels are elevated in patients with a variety of malignant cancer types [13]. However, the production of hepcidin is not limited to the liver, but the tumor tissue itself might contribute to the rise in hepcidin seen in cancer. Furthermore, dysregulated hepcidin may have local impacts on tumor growth and the potential for malignancy in addition to altering systemic iron homeostasis because of its capacity to increase iron retention in cells [14, 15].
Serum ferritin and hepcidin may play a key role in the development of prostate cancer, but little is known about their interaction. The results of this study could lead to the development of novel and/or supplementary diagnostic methods that would increase diagnostic accuracy while reducing the need for needless tests, biopsies, and treatments.
2. Materials and Methods
2.1. Study Design/Site
This case‐control study was conducted at the Methodist Hospital, Wenchi, a Christian faith‐based municipal hospital in Bono Region of Ghana between the periods of June 2022 to October 2022. The hospital, which has 238 beds, serves as a referral hub for 20 public and private healthcare facilities in the Wenchi municipality. According to the 2021, Population and Housing Census, Wenchi Municipal has a population of 89,739 people and mostly engaged in farming [16].
2.2. Ethical Consideration
Ethical approval was sought from the Research and Ethics Committee of the University for Development Studies, Tamale, Ghana (UDS/RB/072/22). Permission was sought from the management of Methodist Hospital Wenchi. Written informed consent was taken from the study participants.
2.3. Study Population and Sample Size
The study involved 90 consenting participants who were categorized into three groups as follows: 30 men diagnosed with prostate cancer, 30 with BPE, and 30 apparently healthy age‐matched men without any prostate‐related disorders as a control group.
2.4. Laboratory Methods
Blood samples were drawn under aseptic conditions into serum separator and K3EDTA coated containing vacutainer tubes, respectively. The K3EDTA blood was used for full blood count analysis using three‐part Mindray BC‐3000 Plus hematology autoanalyzer. The gel tube blood was allowed to clot and centrifuged at 3000 rpm/5 min to obtain the serum. The serum was separated into Eppendorf tubes and stored at –80◦C until assayed. The stored serum was subsequently used for the determination of ferritin and hepcidin measurement using sandwich ELISA.
2.5. Data Analysis
Data were entered into Microsoft Word Excel 2013 and analyzed with Statistical Package for the Social Sciences (SPSS), version 27. Test for normality was done with a box plot and Kolmogorov–Smirnov test. Results were presented in tables and figures. Parametric data was presented as means and standard deviations, whilst non‐parametric data was presented as median and Inter‐quartile range. Continuous variables within the three groups were compared using one‐way ANOVA for parametric data and Kruskal–Wallis for non‐parametric data. Post hoc was used to compare within groups. Correlation of parametric and non‐parametric data were generated by Pearsons's and Spearman correlation, respectively. p < 0.05 was deemed statistically significant.
3. Results
3.1. Demographic and Complete Blood Cell Parameters of Study Participants
Table 1 shows the demographic and complete blood cell parameters of the study participants as stratified by prostate disorders and controls. Hemoglobin (p < 0.001), RBC (p < 0.001), and HCT (p = 0.018) were significantly lower in patients with prostate cancers compared to those with BPE and the normal control group. MCHC showed significantly lower levels in prostate cancer cases compared to the controls (32.65 ± 0.89 vs. 32.01 ± 1.26, p = 0.032). RDW‐CV was significantly higher in prostate cancer cases compared to BPE and the control subjects [14.70 (14.45–15.40) vs. 14.30 (13.78–14.70), p = 0.027; 14.70 (14.45–15.40) vs. 14.50 (13.70–14.80), p = 0.045]. TWBC was higher in prostate patients than in controls (p = 0.004). However, within groups, a significant difference was observed between prostate cancer patients and controls [6.6 (5.3–7.9) vs. 5.1 (4.7–6.2)] as well as between BPE patients and controls [5.5 (5.2–6.9) vs. 5.1 (4.7–6.2)]. The remaining parameters: MCV, MCH, and platelets showed no significant difference between groups (p > 0.05).
Table 1.
Demographic and complete blood cell parameters of the study participants.
| Variables | Participants | p value within groups | Significant pairs | ||
|---|---|---|---|---|---|
| Healthy men (%) (n = 30)a | BPE (%) (n = 30)b | Prostate Cancer (30)c | |||
| Age (years) | 67.0 (62.3–75.0) | 67.0 (60.8–72.0) | 69.0 (67.0–74) | 0.169 | |
| Hb (g/dL) | 14.9 (13.5–15.7) | 12.8 (12.5–13.9) | 11.9 (9.5–13.3) | < 0.001 | a & b, a & c |
| RBC × 109/L | 5.0 (4.5–5.3) | 4.3 (4.0–5.0) | 4.2 (3.3–4.7) | < 0.001 | a & b, a & c, b & c |
| HCT (%) | 44.7 (42.7–47.6) | 39.8 (37.7– 43.3) | 41.2 (37.0–43.9 | 0.018 | a & b, a & c, b & c |
| MCV (fL) | 91.3 ± 4.7 | 90.9 ± 9.31 | 90.4 ± 6.4 | 0.435 | |
| MCH (pg) | 30.2 (29.3–30.9) | 29.6 (28.1–31.0) | 28.8 (27.2–30.9) | 0.122 | |
| MCHC (g/dL) | 32.7 ± 0.9 | 32.6 ± 0.93 | 32.01 ± 1.26 | 0.032 | a & c |
| RDW‐CV (%) | 14.3 (13.8–14.7) | 14.5 (13.7–14.8) | 14.7 (14.5–15.4) | 0.045 | a & c, b & c |
| TWBC × 109/L | 5.1 (4.7–6.2) | 5.5 (5.2–6.9) | 6.6 (5.3–7.9) | 0. 004 | a & b, a & c |
| Platelet × 109/L | 256.5 (214.0–296.5) | 234.0 (207.3–333.0) | 233.0 (223.3–257.8) | 0.837 | |
Note: Parametric data (presented in means ± standard deviation) was generated by one‐way ANOVA, and non‐parametric data (presented in medians (25th–75th percentile)) generated by Mann Whitney U Test, p < 0.05 was considered statistically significant.
Abbreviations: fL = femtoliter, g/dL = grams per deciliter, Hb = hemoglobin, HCT = hematocrit, MCV = mean cell volume, MCH = mean corpuscular hemoglobin, MCHC = mean corpuscular hemoglobin concentration, RDW‐CV = red cell distribution width‐coefficient of variation, WBC = white blood cell.
3.2. Serum PSA Concentration Among Study Participants With Prostrate Disorders
The figure below shows the serum PSA concentration variations between patients with BPE and prostate cancer. Levels of PSA were significantly higher among the prostate cancer patients compared to BPE patients [32.1 (18.2–47.6) vs. 18.3 (12.2–20.6), p < 0.001] (Figure 1).
Figure 1.

Serum PSA concentration among the study participants with prostrate disorders; ng/mL = nanogram per milliliter. Data are presented as median (IQR = Interquartile ranges) and Mann–Whitney U test was used to compare the levels of PSA among the participants. p < 0.05 was considered statistically significant.
3.3. Serum Levels of Ferritin Among the Study Participants
Figure 2 illustrates the serum levels of ferritin among the study participants. Among the 90 study participants, serum ferritin levels were significantly higher in the patients with prostate cancer compared to those with BPE as well as the normal control group; [239.6 (208.8–357.1) vs. 206.7 (175.6–259.5) vs. 123.7 (103.0–144.4), p = 0.001], respectively.
Figure 2.

Serum ferritin levels among the study participants stratified by prostrate disorders and controls. ng/dL = nanogram per deciliter; BPE = benign prostatic enlargement. Data are presented as median (IQR = interquartile ranges) and Kruskal–Wallis test was used to compare the levels of ferritin among the participants. p < 0.05 was considered statistically significant.
3.4. Serum Hepcidin Levels Among the Study Participants
Figure 3 serum hepcidin levels among the study participants. The serum levels of hepcidin were significantly higher in patients with Prostate Cancer than those with BPE and the normal control group: [33.4 (29.2–36.6) vs. 25.8 (19.7–29.8) vs. 21.1 (17.8–25.2), p = 0.001], respectively (Figure 3).
Figure 3.

Serum hepcidin levels among the study participants. BPE = benign prostatic enlargement; ng/mL = nanogram per milliliter. Data are presented as median (IQR = interquartile ranges), and Kruskal–Wallis test was used to compare the levels of hepcidin among the participants. p < 0.05 was considered statistically significant.
3.5. Correlation of PSA With Levels of Ferritin and Hepcidin Among the Study Participants
Figure 4 illustrates the correlation PSA with levels of ferritin and hepcidin among the study participants. Levels of PSA strongly correlated with serum ferritin (r = 0.739, p = < 0.001), whereas a moderate positive correlation was observed between levels of PSA and serum hepcidin among the participants (r = 0.670, p = < 0.001).
Figure 4.
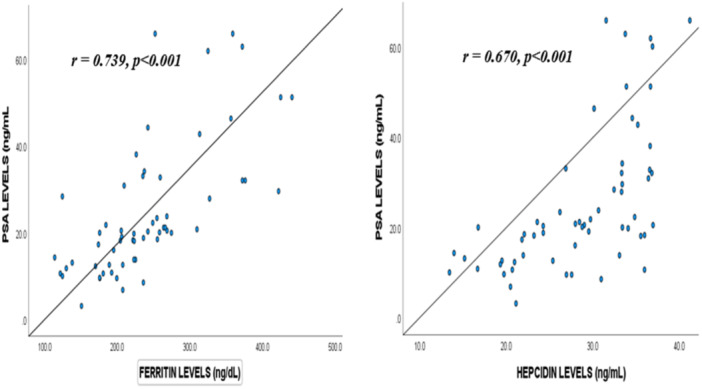
Correlation of serum PSA with levels of ferritin and hepcidin among the study participants. ng/dL = nanogram per deciliter; ng/mL = nanogram per milliliter; PSA = prostate‐specific antigen; r = Correlation coefficient. Correlation was assessed by Spearman rank correlation test and p < 0.05 was considered statistically significant.
3.6. Diagnostic Value of Serum Ferritin and Hepcidin as Biomarkers for Prostate Disorders
Figure 5 represents ROC analysis showing the diagnostic value of serum ferritin, hepcidin, and PSA ratios as markers for prostate cancer (Figure 5A) and BPE (Figure 5B). At optimum cut‐off points of serum ferritin (311.6 ng/dL), and hepcidin (29.6 ng/mL), PSA (28.2 ng/mL), PSA/ferritin ratio (0.115), and PSA/hepcidin ratio (0.865) yielded an area under the curve (AUC) values of 0.687, 0.777, 0.764, 0.724, and 0.711, respectively for prostate cancer. The sensitivities and specificities were 33.3% and 96.7% for ferritin, 76.7% and 76.7% for hepcidin, 63.3% and 100% for PSA, 56.7% and 96.7% for PSA/ferritin ratio and 60% and 86.7% for PSA/hepcidin ratio, respectively (Figure 5B). For BPE, we found reduced AAUC for all the parameters.
Figure 5.

Diagnostic values of serum ferritin, hepcidin, and PSA ratios as biomarkers for prostate cancer (A) and BPE (B). AUC = area under the curve, BPE = benign prostatic enlargement, ROC = receive operating curve.
4. Discussion
In Ghana, the incidence of prostate neoplasm and its associated mortality is on the rise [3]. Total PSA remains the most widely used diagnostic biomarker for prostate cancer (PCa), but it lacks sensitivity and specificity, which leads to unnecessary biopsies and mismanagements. This study investigated the correlation of serum PSA with ferritin and hepcidin as a complementary diagnostic biomarker for prostate cancers.
The study found a significantly higher serum PSA levels among prostate cancer patients compared to the BPE group, which agrees with earlier studies [17, 18, 19]. This could be attributed to the disruption of prostatic acini that secrete PSA in prostate cancer. Again, malignant cells allow PSA to pass easily through the cell wall into the surrounding extracellular fluid and eventually reach the bloodstream due to lack of basal cell membrane by the malignant prostate cells thereby raising blood PSA levels [20].
Serum ferritin presents a paradox, as a protein for storage of iron as well as an inflammatory marker. Ferritin plays a number of important roles in many physiological and pathological processes, including malignancies [7]. Our study found a significantly higher level of ferritin in PCa patients compared to those with BPE as well as the normal control group. This finding is consistent with a previous study conducted by Wang et al. Again, the high levels of serum ferritin observed in patients with prostate disorders were further highlighted by a strong positive correlation with increased PSA levels, which is in consonance with studies conducted in Uganda and China, respectively [8, 19]. The plausible reason for this finding could be attributed to the fact that ferritin is an acute phase protein not only produced by the hepatocytes but may also be produced by the prostate cancer cells making them partly independent of external iron supply. In contrast to our finding, a study by Kuvibidila and co reported an inverse relationship between serum ferritin levels and the disease stage of prostate cancer [21]. The variations in the findings may be related to the differences in the study design and geographical locations. While our study employed prostate cancer patients on therapy in Ghana, their study recruited untreated prostate cancer patients in New Orleans.
Hepcidin, a peptide hormone primarily produced in the liver, plays a significant role in iron homeostasis by regulating iron metabolism in the body. Elevated hepcidin levels have been linked to chronic inflammation, cancer‐related anemia, and iron sequestration in various malignancies [10]. Hepcidin is thought to have a role in the development of prostate cancer by lowering ferroportin expression and raising intracellular iron levels, allowing cancer cells to proliferate, migrate, and resist apoptosis [13]. The study observed that prostate cancer patients exhibited significantly higher levels of hepcidin than those with BPE and healthy individuals, suggesting a possible role of hepcidin in tumor progression and iron dysregulation in the cancer process. This is consistent with findings by Zhao and coleagues in 2018 [13]. This finding could probably be due to the consequence of the inflammatory cytokines such as interleukin 6 that cause an upregulation of hepcidin not limited to the liver but also in prostate cancer tissues. The dysregulated hepcidin may have local impacts on tumor growth and the potential for malignancy in addition to altering systemic iron homeostasis because of its capacity to increase iron retention in cells [15, 22].
The study also found a moderate significant positive correlation between serum hepcidin and increased levels of PSA which corroborates with a study conducted in Peru [23]. This finding can also be attributed to the upregulation of hepcidin caused by the inflammatory cytokines in prostate cancer patients.
While most studies align with the current findings, a study by Alcantara‐Zapata et al. in 2023 found no association between hepcidin levels PSA [23]. This discrepancy could be attributed the differences in the study subjects, while our study recruited individuals with prostate‐elated disorders, Alcantara‐Zapata et al. used healthy population.
This study further assessed the diagnostic values of the serum PSA, ferritin, and hepcidin. Our results suggest that serum PSA, ferritin, and hepcidin may serve as a possible diagnostic marker for prostate cancer which is consistent with earlier studies conducted in China [7, 8]. The current finding is in line with a study by Wang and colleagues, and this could be attributed to hyperexpression of hepcidin by the prostate cancer cells, known to regulate cell proliferation, migration, and apoptosis by increasing intracellular iron transportation [24].
On the other hand, the serum ferritin and hepcidin among the BPE cohort yielded a low area under the curve with low sensitivity and specificity required for BPE diagnosis.
Given that PSA alone may not provide sufficient sensitivity or specificity for diagnosing prostate cancer, the literature suggests the need for developing a feasible multivariable model to complement its diagnostic and prognostic performance [7]. By comparing the performance of the ratios of PSA/ferritin and PSA/hepcidin combined markers yielded no improved sensitivity and specificity over that of the PSA alone. This is in contrast with a study by Wang et al., which provides the additional sensitivity and specificity needed to improve the diagnostic and prognostic value of the PSA test particularly in patients with hyperferritinemia. The difference in findings could be attributed to the variation in sample size and study subjects.
Although it is widely acknowledged that cancer development has a hereditary basis, there is however a growing evidence that the host inflammatory response is linked to cancer growth and progression [25]. This systemic inflammation causes derangement in the numbers and composition of circulating blood cell indices such as the red blood cells, white blood cells, and platelets [26]. This study found a significant reduction in the concentrations of hemoglobin, number of red blood cell, hematocrit, mean cell hemoglobin concentration with elevated levels of total white blood cells, and RDW‐CV in subjects with prostate disease group compared to the controls. This current study agrees well with previous studies [1, 27].
However, our finding is in disparity with a study by Cihan colleagues that reported low white blood cell count among prostate disease patients [28]. The reduced haemogram (anemia) could be attributed to the inhibition of erythropoiesis by inflammatory cytokines such as IL‐1 and TGF‐β as well as up regulatory activity of hepcidin caused by IL‐6 leading to ineffective erythropoiesis and shortening of erythrocyte life span. It could also be due to the impaired function of the bone marrow by infiltrated malignant cells, and the cytotoxic effect of the medications used in cancer treatment of prostate disorders accounts for the anemia [29]. The superimposed infections experienced in prostate‐related disorders may be the plausible reason for the higher leukocyte count found among prostate cancer patients.
The higher RDW‐CV % observed in participants with prostate cancer than in BPE and controls could probably result from deficiency of a nutrient, including iron deficiency or compensatory to the ongoing anemia. This finding agrees with a study conducted in Asia [30]. The study could not link the levels of ferritin and hepcidin to the histopathological findings such as the Gleason score. Again, the study is limited to a single‐center and findings from this current study were restricted to prostate disease patients on therapy and may not reflect untreated prostate disease patients.
5. Conclusions
Serum levels of ferritin and hepcidin were significantly higher in prostate cancer patients compared to BPE and control group. Serum PSA levels were found to be higher among patients with prostate cancer compared to those with BPE. High levels of serum ferritin and hepcidin significantly correlated with increased serum total PSA levels and may serve as a possible marker to complement the noninvasive total PSA to improve diagnostic accuracy of prostate cancer and BPE. A multicenter study with a larger cohort to include untreated prostate cancer patients is recommended to elucidate the diagnostic and prognostic value of ferritin and hepcidin as complementary biomarkers of prostate cancer. Also, exploring additional inflammatory markers (such as IL‐6, CRP, or TNF‐α) that might influence the levels of ferritin and hepcidin would be more helpful to validate these findings.
Author Contributions
Samuel Kwasi Appiah: conceptualization, investigation, methodology, validation, visualization, writing – review and editing, writing – original draft, project administration, formal analysis, data curation, supervision, resources. Bosomtwi Boateng: conceptualization, data curation, supervision, writing – review and editing. Charles Nkansah: writing – review and editing, formal analysis, software. Bismark Nantomah: writing – original draft, writing – review and editing, supervision. Sibiri Ballu: writing – review and editing, supervision. Gabriel Abbam: investigation, writing – review and editing, data curation. Samira Daud: methodology, validation, visualization, writing – review and editing. Gifty Yambor Yenpiini: data curation, resources, writing – original draft, investigation. Ethel Akabawon Abagulum: data curation, resources, investigation. Felix Osei‐Boakye: formal analysis, software, writing – review and editing, validation. Christopher Nkrumah: investigation, data curation, writing – review and editing. Michael Asamoah Gyamfi: methodology, writing – review and editing, validation. Eric Antwi Osei Kwadwo: investigation, methodology, writing – review and editing. Yussif Adams: writing – review and editing, supervision, formal analysis. Simon Bannison Bani: project administration, methodology, writing – review and editing. Moses Banyeh: software, formal analysis, supervision, writing – review and editing. Adams Abubakari Sadick: data curation, resources, investigation. Haajaratu Zama Alhassan: investigation, data curation, resources. Solomon Yeboah: investigation, data curation, methodology. Charles A. Derigubah: visualization, validation, writing – review and editing. Ejike Felix Chukwurah: supervision, writing – review and editing, validation.
Conflicts of Interest
The authors declare no conflicts of interest.
Transparency Statement
The lead author Samuel Kwasi Appiah affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Acknowledgments
The authors appreciate the enormous contributions of the management and staff of Methodist Hospital Wenchi for their immense support. We appreciate the contributions of all participants in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Zhao P., Zhu Z., and Zhang X., “Hematological Markers and Prostate Cancer Risk: A Mendelian Randomization Study,” Research Square (2023): 1–14, http://10.21203/rs.3.rs-2815251/v1. [Google Scholar]
- 2. Bray F., Laversanne M., Sung H., et al., “Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians 74, no. 3 (2024): 229–263, 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 3. Yeboah E. D., “Prevalence of Benign Prostatic Hyperplasia and Prostate Cancer in Africans and Africans in the Diaspora,” Journal of the West African College of Surgeons 6 (2016): 1–30. [PMC free article] [PubMed] [Google Scholar]
- 4. Black A. and Berg C. D., “Prostate‐Specific Antigen Screening for Prostate Cancer in Older Men in the United States of America,” Gerontology 58, no. 4 (2012): 331–336, 10.1159/000334242. [DOI] [PubMed] [Google Scholar]
- 5. Stamey T. A., Caldwell M., McNeal J. E., Nolley R., Hemenez M., and Downs J., “The Prostate Specific Antigen Era in the United States Is over for Prostate Cancer: What Happened in the Last 20 Years?,” Journal of Urology 172, no. 4 Part 1 (2004): 1297–1301, 10.1097/01.ju.0000139993.51181.5d. [DOI] [PubMed] [Google Scholar]
- 6. Walter S. D., Hu J., Talala K., Tammela T., Taari K., and Auvinen A., “Estimating the Rate of Overdiagnosis With Prostate Cancer Screening: Evidence From the Finnish Component of the European Randomized Study of Screening for Prostate Cancer,” Cancer Causes & Control 32, no. 11 (2021): 1299–1313, 10.1007/s10552-021-01480-8. [DOI] [PubMed] [Google Scholar]
- 7. Wang X., An P., Zeng J., et al., “Serum Ferritin in Combination With Prostate‐Specific Antigen Improves Predictive Accuracy for Prostate Cancer,” Oncotarget 8, no. 11 (2017): 17862–17872, 10.18632/oncotarget.14977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Su Q., Lei T., and Zhang M., “Association of Ferritin With Prostate Cancer,” Journal of B.U.ON.: Official Journal of the Balkan Union of Oncology 22, no. 3 (2017): 766–770. [PubMed] [Google Scholar]
- 9. Ghosh S., Dawn I., and Kundu S. K., “Assessment of Serum Ferritin Along With Serum PSA in Differential Diagnosis of Benign Prostatic Hypertrophy and Prostate Cancer,” Journal of Cardiovascular Disease Research 13, no. 5 (2022): 1628–1634. [Google Scholar]
- 10. Joachim J. H. and Mehta K. J., “Hepcidin in Hepatocellular Carcinoma,” British Journal of Cancer 127, no. 2 (2022): 185–192, 10.1038/s41416-022-01753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pinnix Z. K., Miller L. D., Wang W., et al., “Ferroportin and Iron Regulation in Breast Cancer Progression and Prognosis,” Science Translational Medicine 2, no. 43 (2010): 43ra56, 10.1126/scisignal.3001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ako E., Porter J. B., and Walker J. M., “Cardiovascular Complications in Sickle Cell Disease,” Journal of the American College of Cardiology 65, no. 10 (2015): A1339, 10.1016/S0735-1097(15)61339-0. [DOI] [Google Scholar]
- 13. Zhao B., Li R., Cheng G., et al., “Role of Hepcidin and Iron Metabolism in the Onset of Prostate Cancer,” Oncology Letters 15, no. 6 (2018): 9953–9958, 10.3892/ol.2018.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng Q., He B., Liu X., et al., “Prognostic Value of Pre‐Operative Inflammatory Response Biomarkers in Gastric Cancer Patients and the Construction of a Predictive Model,” Journal of Translational Medicine 13 (2015): 66, 10.1186/s12967-015-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun C. C., Vaja V., Babitt J. L., and Lin H. Y., “Targeting the Hepcidin‐Ferroportin Axis to Develop New Treatment Strategies for Anemia of Chronic Disease and Anemia of Inflammation,” American Journal of Hematology 87, no. 4 (2012): 392–400, 10.1002/ajh.23110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. GSS , Ghana 2021 Population and Housing Census General Report 3A, 2021.
- 17. Bansal R., “High Levels of the PSA (Prostate Specific Antigen) in Patients With Benign Prostatic Hyperplasia and Prostate Cancer Abstract,” Prostate Cancer Arch Cancer Research 8, no. 4 (2020): 14, www.acanceresearch.com. [Google Scholar]
- 18. Gretzer M. B. and Partin A. W., “PSA Levels and the Probability of Prostate Cancer on Biopsy,” European Urology Supplements 1, no. 6 (2002): 21–27, 10.1016/S1569-9056(02)00053-2. [DOI] [Google Scholar]
- 19. Okuja M., Ameda F., Dabanja H., Bongomin F., and Bugeza S., “Relationship Between Serum Prostate‐Specific Antigen and Transrectal Prostate Sonographic Findings in Asymptomatic Ugandan Males,” African Journal of Urology 27, no. 1 (2021): 58, 10.1186/s12301-021-00162-w. [DOI] [Google Scholar]
- 20. el Farhaoui H., Jdaini A., Elabbassi O., Bounouar O., Elmoudane A., and Barki A., “Management of a Localized Prostatic Adenocarcinoma Despite the Very High Rate of PSA and the Large Tumor Mass: Does PSA Level Indicate the Stage of Prostate Cancer?,” Radiology Case Reports 18, no. 10 (2023): 3501–3503, 10.1016/j.radcr.2023.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuvibidila S. R., Gauthier T., and Rayford W., “Serum Ferritin Levels and Transferrin,” Journal of the National Medical Association 96, no. 5 (2004): 641–649. [PMC free article] [PubMed] [Google Scholar]
- 22. Lee S. W., Kim Y. H., Chung W., et al., “Serum Hepcidin and Iron Indices Affect Anemia Status Differently According to the Kidney Function of Non‐Dialysis Chronic Kidney Disease Patients: Korean Cohort Study for Outcome in Patients With Chronic Kidney Disease (KNOW‐CKD),” Kidney and Blood Pressure Research 42 (2017): 1183–1192, 10.1159/000485865. [DOI] [PubMed] [Google Scholar]
- 23. Alcantara‐Zapata D. E., Thiersch M., and Gonzales G. F., “Association of Serum Hepcidin With Prostate‐Specific Antigen Levels in Men From High Andean Cities of Peru,” International Journal of Health Sciences 17, no. 2 (2023): 28–36, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9986881/. [PMC free article] [PubMed] [Google Scholar]
- 24. Wang F., Liu A., Bai R., et al., “Hepcidin and Iron Metabolism in the Pathogenesis of Prostate Cancer,” Journal of B.U.ON.: Official Journal of the Balkan Union of Oncology 22, no. 5 (2017): 1328–1332. [PubMed] [Google Scholar]
- 25. Zhao H., Wu L., Yan G., et al., “Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention,” Signal Transduction and Targeted Therapy 6, no. 1 (2021): 263, 10.1038/s41392-021-00658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pinato D. J., Shiner R. J., Seckl M. J., Stebbing J., Sharma R., and Mauri F. A., “Prognostic Performance of Inflammation‐Based Prognostic Indices in Primary Operable Non‐Small Cell Lung Cancer,” British Journal of Cancer 110, no. 8 (2014): 1930–1935, 10.1038/bjc.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Watts E. L., Perez‐Cornago A., Kothari J., Allen N. E., Travis R. C., and Key T. J., “Hematologic Markers and Prostate Cancer Risk: A Prospective Analysis in UK Biobank,” Cancer Epidemiology, Biomarkers & Prevention 29, no. 8 (2020): 1615–1626, 10.1158/1055-9965.EPI-19-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cihan Y. B., Arslan A., and Ergul M. A., “Subtypes of White Blood Cells in Patients With Prostate Cancer or Benign Prostatic Hyperplasia and Healthy Individuals,” Asian Pacific Journal of Cancer Prevention 14, no. 8 (2013): 4779–4783, 10.7314/APJCP.2013.14.8.4779. [DOI] [PubMed] [Google Scholar]
- 29. Fernandes J. V., Cobucci R. N., Jatobá C. A. et al., “The Role of the Mediators of Inflammation in Cancer Development,” Pathology Oncology Research: POR 21, no. 3 (2015): 527–534, 10.1007/s12253-015-9913-z. [DOI] [PubMed] [Google Scholar]
- 30. Albayrak S., Zengin K., Tanik S., Bakirtas H., Imamoglu A., and Gurdal M., “Red Cell Distribution Width as a Predictor of Prostate Cancer Progression,” Asian Pacific Journal of Cancer Prevention 15, no. 18 (2014): 7781–7784, 10.7314/APJCP.2014.15.18.7781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


