Abstract
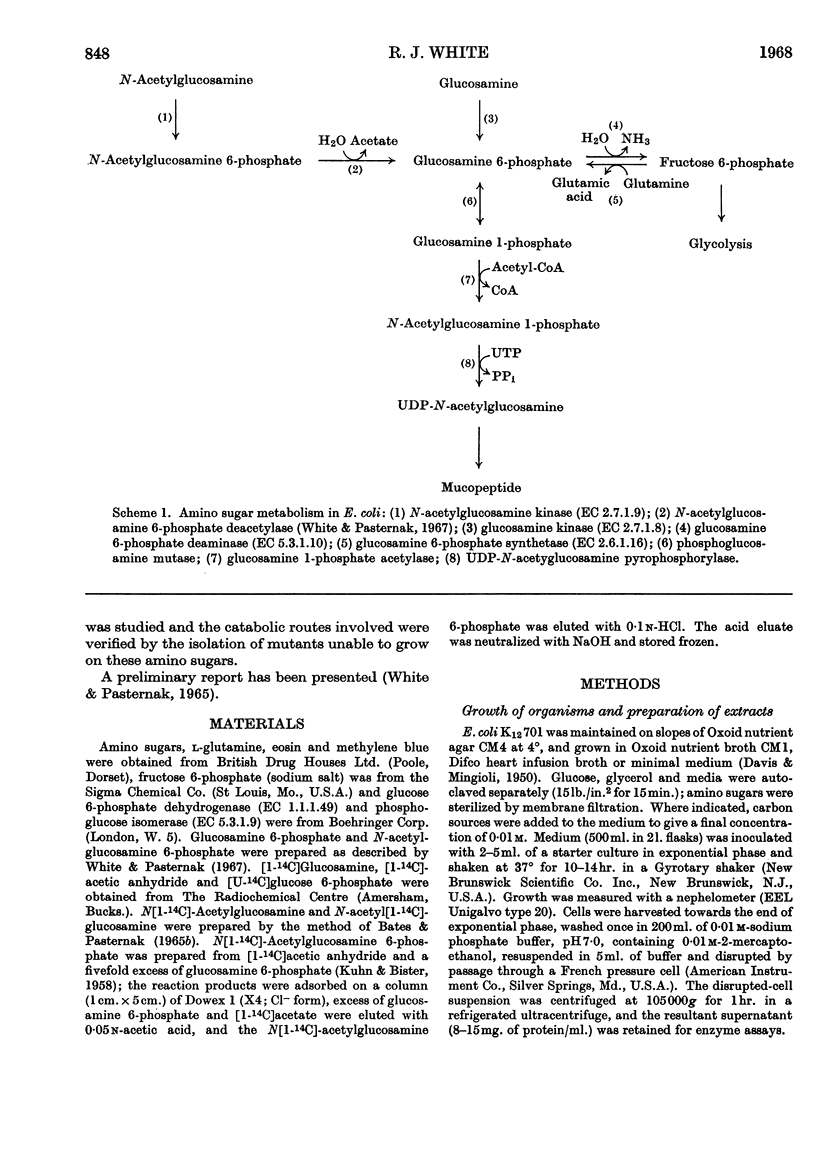
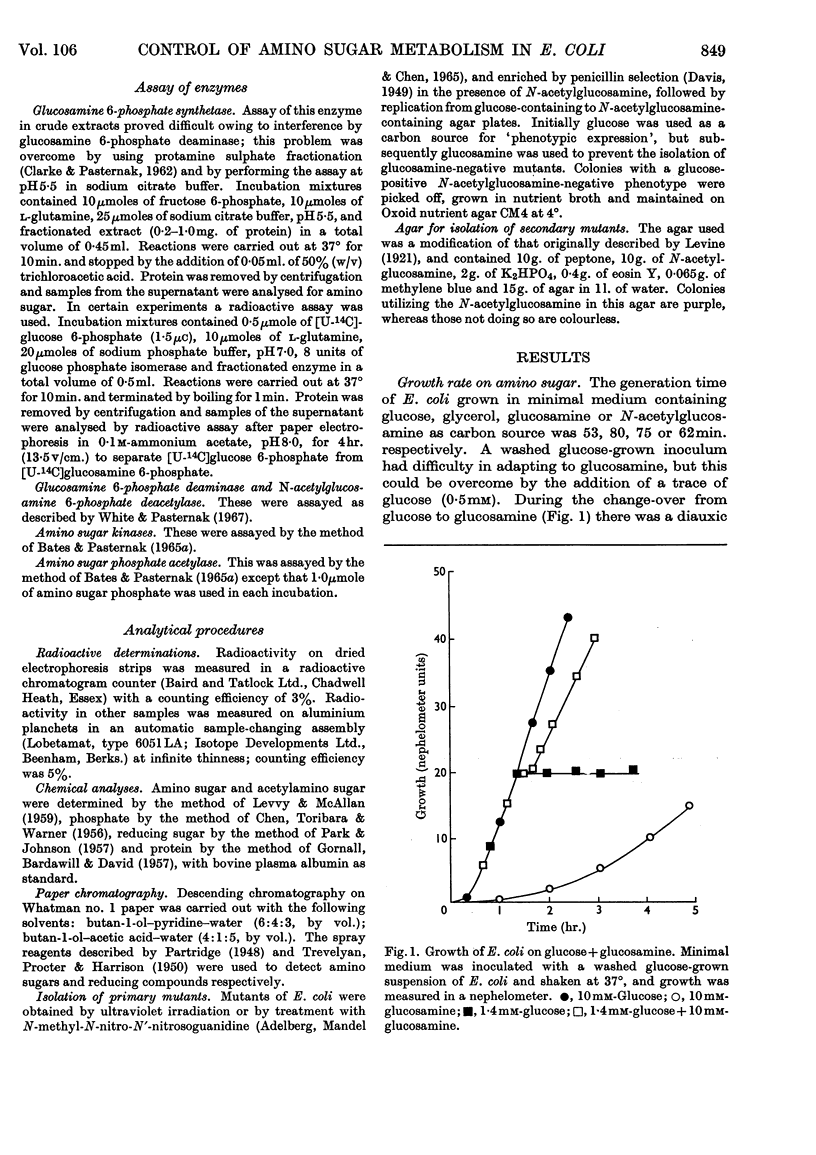
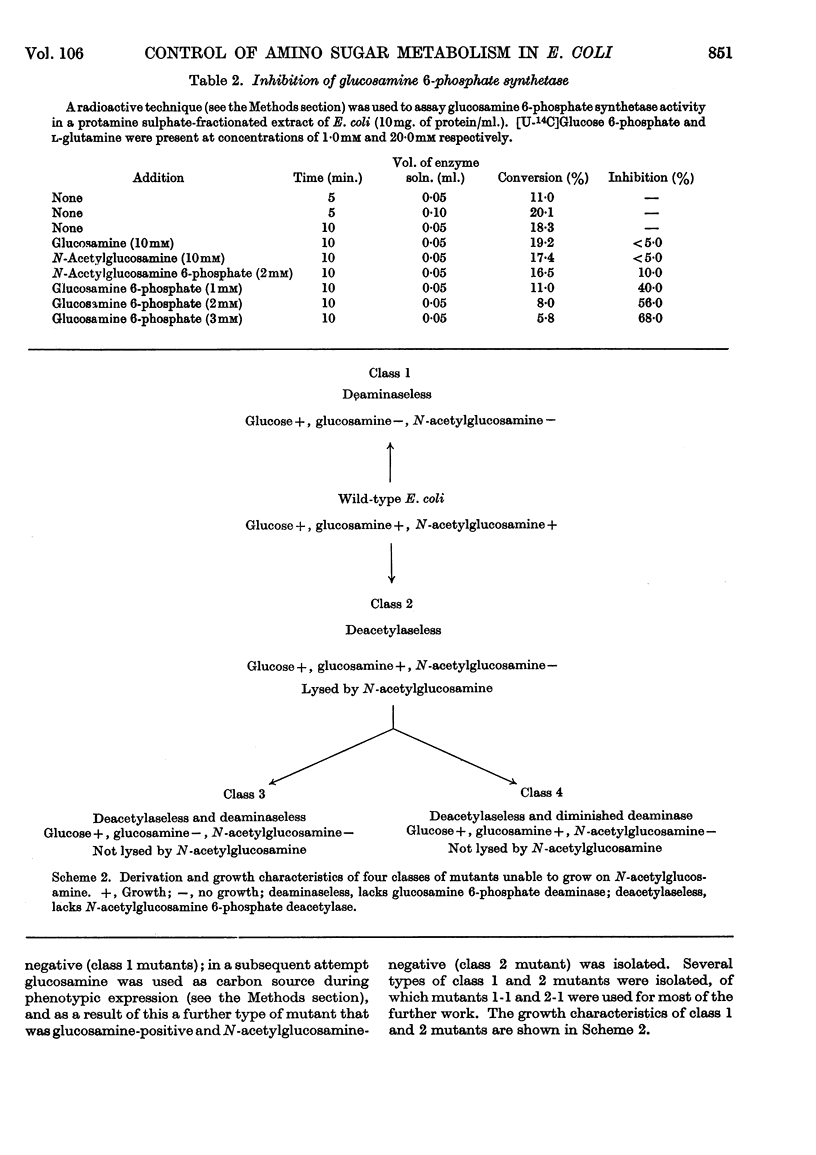
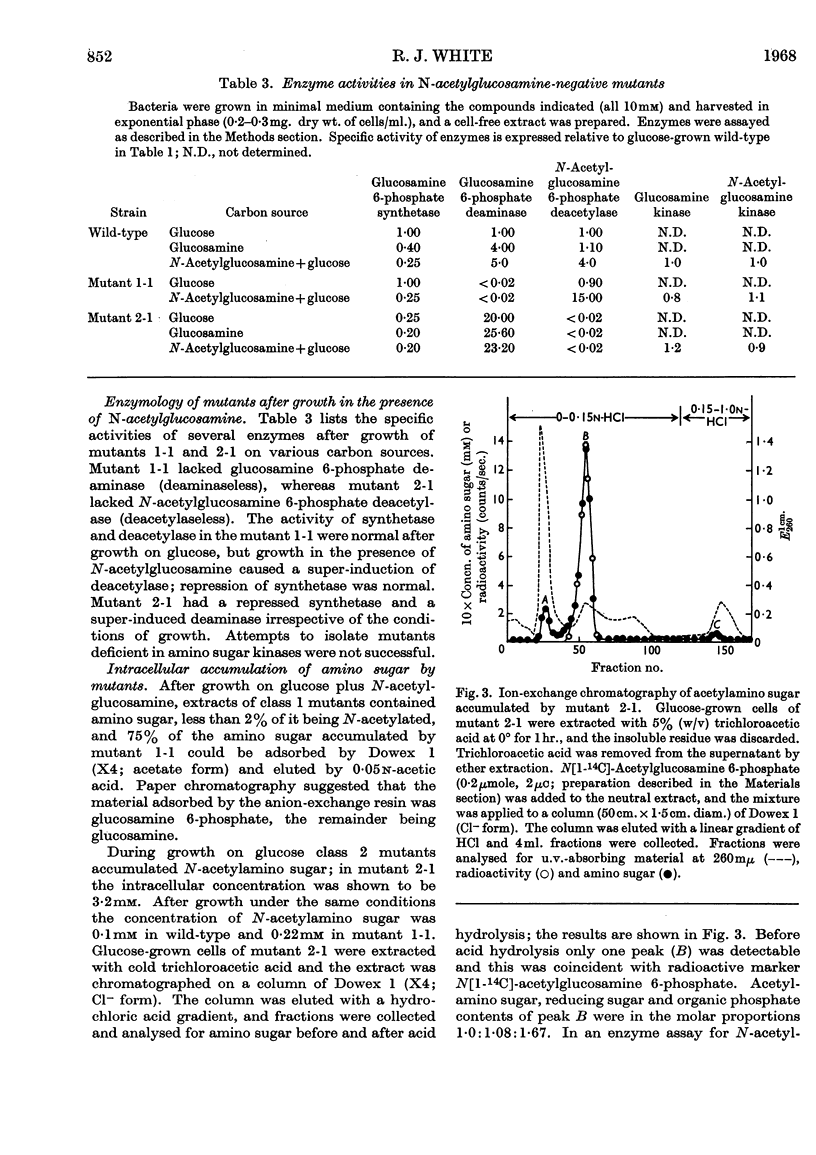
1. Growth of Escherichia coli on glucosamine results in an induction of glucosamine 6-phosphate deaminase [2-amino-2-deoxy-d-glucose 6-phosphate ketol-isomerase (deaminating), EC 5.3.1.10] and a repression of glucosamine 6-phosphate synthetase (l-glutamine–d-fructose 6-phosphate aminotransferase, EC 2.6.1.16); glucose abolishes these control effects. 2. Growth of E. coli on N-acetylglucosamine results in an induction of N-acetylglucosamine 6-phosphate deacetylase and glucosamine 6-phosphate deaminase, and in a repression of glucosamine 6-phosphate synthetase; glucose diminishes these control effects. 3. The synthesis of amino sugar kinases (EC 2.7.1.8 and 2.7.1.9) is unaffected by growth on amino sugars. 4. Glucosamine 6-phosphate synthetase is inhibited by glucosamine 6-phosphate. 5. Mutants of E. coli that are unable to grow on N-acetylglucosamine have been isolated, and lack either N-acetylglucosamine 6-phosphate deacetylase (deacetylaseless) or glucosamine 6-phosphate deaminase (deaminaseless). Deacetylaseless mutants can grow on glucosamine but deaminaseless mutants cannot. 6. After growth on glucose, deacetylaseless mutants have a repressed glucosamine 6-phosphate synthetase and a super-induced glucosamine 6-phosphate deaminase; this may be related to an intracellular accumulation of acetylamino sugar that also occurs under these conditions. In one mutant the acetylamino sugar was shown to be partly as N-acetylglucosamine 6-phosphate. Deaminaseless mutants have no abnormal control effects after growth on glucose. 7. Addition of N-acetylglucosamine or glucosamine to cultures of a deaminaseless mutant caused inhibition of growth. Addition of N-acetylglucosamine to cultures of a deacetylaseless mutant caused lysis, and secondary mutants were isolated that did not lyse; most of these secondary mutants had lost glucosamine 6-phosphate deaminase and an uptake mechanism for N-acetylglucosamine. 8. Similar amounts of 14C were incorporated from [1-14C]-glucosamine by cells of mutants and wild-type growing on broth. Cells of wild-type and a deaminaseless mutant incorporated 14C from N-acetyl[1-14C]glucosamine more efficiently than from N[1-14C]-acetylglucosamine, incorporation from the latter being further decreased by acetate; cells of a deacetylaseless mutant showed a poor incorporation of both types of labelled N-acetylglucosamine.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATES C. J., PASTERNAK C. A. FURTHER STUDIES ON THE REGULATION OF AMINO SUGAR METABOLISM IN BACILLUS SUBTILIS. Biochem J. 1965 Jul;96:147–154. doi: 10.1042/bj0960147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATES C. J., PASTERNAK C. A. THE INCORPORATION OF LABELLED AMINO SUGARS BY BACILLUS SUBTILIS. Biochem J. 1965 Jul;96:155–158. doi: 10.1042/bj0960155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE J. S., PASTERNAK C. A. The regulation of amino sugar metabolism in Bacillus subtilis. Biochem J. 1962 Jul;84:185–191. doi: 10.1042/bj0840185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMB D. G., ROSEMAN S. Glucosamine metabolism. IV. Glucosamine-6-phosphate deaminase. J Biol Chem. 1958 Jun;232(2):807–827. [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORFMAN A. Metabolism of the mucopolysaccharides of connective tissue. Pharmacol Rev. 1955 Mar;7(1):1–31. [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Galactose-sensitive mutants of Salmonella. II. Bacteriolysis induced by galactose. Biochim Biophys Acta. 1961 Apr 15;48:470–483. doi: 10.1016/0006-3002(61)90045-2. [DOI] [PubMed] [Google Scholar]
- INGRAM V. M., SALTON M. R. The action of fluorodinitrobenzene on bacterial cell walls. Biochim Biophys Acta. 1957 Apr;24(1):9–14. doi: 10.1016/0006-3002(57)90139-7. [DOI] [PubMed] [Google Scholar]
- KORNFELD S., GLASER L. The synthesis of thymidine-linked sugars. v. thymidine diphosphate-amino sugars. J Biol Chem. 1962 Oct;237:3052–3059. [PubMed] [Google Scholar]
- KORNFELD S., KORNFELD R., NEUFELD E. F., O'BRIEN P. J. THE FEEDBACK CONTROL OF SUGAR NUCLEOTIDE BIOSYNTHESIS IN LIVER. Proc Natl Acad Sci U S A. 1964 Aug;52:371–379. doi: 10.1073/pnas.52.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMBERT R., SAITO Y., VEERKAMP J. H. INCORPORATION OF LABELED DERIVATIVES OF 2-DEOXY-2-AMINO-D-GLUCOSE INTO THE CELL WALLS OF LACTOBACILLUS BIFIDUS VAR. PENNSYLVANICUS. Arch Biochem Biophys. 1965 May;110:341–345. doi: 10.1016/0003-9861(65)90130-x. [DOI] [PubMed] [Google Scholar]
- LEVVY G. A., MCALLAN A. The N-acetylation and estimation of hexosamines. Biochem J. 1959 Sep;73:127–132. doi: 10.1042/bj0730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W. F., Jr, Magasanik B. Glucose-lactose diauxie in Escherichia coli. J Bacteriol. 1967 Apr;93(4):1397–1401. doi: 10.1128/jb.93.4.1397-1401.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- NAKADA H. I., WOLFE J. B. Glucosamine degradation by Escherichia coli. II. The isomeric conversion of glucosamine 6-PO4 to fructose 6-PO4 and ammonia. Arch Biochem Biophys. 1956 Oct;64(2):489–497. doi: 10.1016/0003-9861(56)90291-0. [DOI] [PubMed] [Google Scholar]
- Partridge S. M. Filter-paper partition chromatography of sugars: 1. General description and application to the qualitative analysis of sugars in apple juice, egg white and foetal blood of sheep. with a note by R. G. Westall. Biochem J. 1948;42(2):238–250. doi: 10.1042/bj0420238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEMAN S. Glucosamine metabolism. I. N-acetylglucosamine deacetylase. J Biol Chem. 1957 May;226(1):115–124. [PubMed] [Google Scholar]
- Schlesinger S., Scotto P., Magasanik B. Exogenous and endogenous induction of the histidine-degrading enzymes in Aerobacter aerogenes. J Biol Chem. 1965 Nov;240(11):4331–4337. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Fraenkel D. G., Lin E. C. The enzymatic lesion of strain MM-6, a pleiotropic carbohydrate-negative mutant of Escherichia coli. Biochem Biophys Res Commun. 1967 Apr 7;27(1):63–67. doi: 10.1016/s0006-291x(67)80040-8. [DOI] [PubMed] [Google Scholar]
- White R. J., Pasternak C. A. The purification and properties of N-acetylglucosamine 6-phosphate deacetylase from Escherichia coli. Biochem J. 1967 Oct;105(1):121–125. doi: 10.1042/bj1050121. [DOI] [PMC free article] [PubMed] [Google Scholar]


