Abstract
Stroke is a main risk to life and fitness in current society, particularly in the aging population. Also, the stroke is recognized as a cerebrovascular accident. It contains a nervous illness, which can result from haemorrhage or ischemia of the brain veins, and regular mains to assorted motor and cognitive damages that cooperate with functionality. Screening for stroke comprises physical examination, history taking, and valuation of risk features like age or certain cardiovascular illnesses. Symptoms and signs of stroke include facial weakness. Even though computed tomography (CT) and magnetic resonance imaging (MRI) are standard diagnosis techniques, artificial intelligence (AI) systems have been constructed based on these methods, which deliver fast detection. AI is gaining high attention and is being combined into numerous areas with medicine to enhance the accuracy of analysis and the quality of patient care. This paper proposes an enhancing neurological disease diagnostics fusion of transfer learning for acute brain stroke prediction using facial images (ENDDFTL-ABSPFI) method. The proposed ENDDFTL-ABSPFI method aims to enhance brain stroke detection and classification models using facial imaging. Initially, the image pre-processing stage applies the fuzzy-based median filter (FMF) model to eliminate the noise in input image data. Furthermore, fusion models such as Inception-V3 and EfficientNet-B0 perform the feature extraction. Moreover, the hybrid of convolutional neural network and bidirectional long short-term memory (CNN-BiLSTM) model is employed for the brain stroke classification process. Finally, the multi‐objective sailfish optimization (MOSFO)-based hyperparameter selection process is carried out to optimize the classification outcomes of the CNN-BiLSTM model. The simulation validation of the ENDDFTL-ABSPFI technique is investigated under the Kaggle dataset concerning various measures. The comparative evaluation of the ENDDFTL-ABSPFI technique portrayed a superior accuracy value of 98.60% over existing methods.
Keywords: Fusion of transfer learning, Acute brain stroke prediction, Facial images, Optimization algorithm, Image pre-processing
Subject terms: Computer science, Information technology
Introduction
Several molecular dysfunctions, like stem-cell exhaustion and genome instability, manifest during ageing and cellular senescence1. Thus, ageing is complexly connected to a spectrum of illnesses, including cancer, stroke, macular degeneration, and more. The ageing process can adversely distress the cerebral vascular and cardiovascular methods, so exacerbating the development of ischemic stroke2. The impairment can raise the risk of stroke in aging populations. Additionally, population analyses have shown a substantial correlation between reduced ischemic stroke and telomere length, emphasizing the vital role of ageing in stroke aetiology. Together, this evidence solidifies stroke as an age-related illness3. Presently, stroke is a main healthcare task. Stroke also called a cerebrovascular chance, is a neurological illness produced by haemorrhage or ischemia of the brain veins and generally causes cognitive impairments and heterogeneous motor, which undermine functionality4. Thus, the early prevention and detection of stroke not only aids global healthy ageing initiatives but also assists in reducing mortality and morbidity among the ageing populations. Timely recognition and improved control of adaptable risk aspects for the prevention of stroke and to evade the future growth of vascular dementia5. Stroke identification includes a comprehensive clinical record, a neurological and physical inspection, and a brain imaging test, for example, an MRI or CT scan, to prevent other stroke mimics and to establish the kind of stroke, its position and the level of brain damage.
A stroke arises once the blood supply to a part of the brain is disturbed, causing brain cells to die of nutrients and absence of oxygen6. The facial signs are also an indication of stroke. Sometimes, weakness is attended by an insensitivity or a struggle to speak. Facial indications include a corner of the mouth, lower lip, or eyelid drooping7. Patients can also struggle to move specific facial muscles, which are more prominent when requested to smile or raise their eyebrows. AI, a computer method focused on mimicking human intelligence, is improving interest and being integrated into multiple domains, including medicine8. Stroke medicine is one of these fields of AI application, which enhances the precision of identification and the excellence of patient care. For stroke management, sufficient investigation of face imaging is essential9. AI models are implemented to decode the data from face imaging and establish a few favourable outcomes. Deep learning (DL) and machine learning (ML) have recently developed and progressed in various applications in various medical care methods. DL and ML-based methodologies are the final technologies that can assist clinical experts in making scientific decisions and forecasts10. In recent decades, multiple analyses have been performed on developing stroke identification utilizing DL and ML relating to speed and accuracy.
This paper proposes an Enhancing Neurological Disease Diagnostics Fusion of Transfer Learning for Acute Brain Stroke Prediction using Facial Images (ENDDFTL-ABSPFI) method. The proposed ENDDFTL-ABSPFI method aims to enhance brain stroke detection and classification models using facial imaging. Initially, the image pre-processing stage applies the fuzzy-based median filter (FMF) model to eliminate the noise in input image data. Furthermore, fusion models such as Inception-V3 and EfficientNet-B0 perform the feature extraction. Moreover, the hybrid of convolutional neural network and bidirectional long short-term memory (CNN-BiLSTM) model is employed for the brain stroke classification process. Finally, the multi‐objective sailfish optimization (MOSFO)-based hyperparameter selection process is carried out to optimize the classification outcomes of the CNN-BiLSTM model. The simulation validation of the ENDDFTL-ABSPFI technique is investigated under the Kaggle dataset concerning various measures. The key contribution of the ENDDFTL-ABSPFI technique is listed below.
The ENDDFTL-ABSPFI model effectively utilizes the FMF technique to mitigate noise in brain images. This pre-processing step improves the quality of input data, ensuring more precise and accurate analysis. By enhancing the signal-to-noise ratio, the FMF method significantly prepares the images for subsequent feature extraction and classification.
The ENDDFTL-ABSPFI method employs Inception-V3 and EfficientNet-B0 techniques for advanced feature extraction, enabling the model to capture complex patterns in brain images. These architectures improve the capability to detect critical features linked to stroke classification. Incorporating these models enhances the overall accuracy and robustness of stroke detection.
The ENDDFTL-ABSPFI approach implements the hybrid CNN-BiLSTM architecture to employ spatial and temporal data. This integration enables improved learning of sequential patterns in brain images for accurate stroke classification. The model enhances stroke detection by capturing intrinsic temporal dependencies and spatial features.
The ENDDFTL-ABSPFI methodology implements the MOSFO model to fine-tune model parameters, balancing multiple objectives to attain optimal performance. This approach improves classification accuracy by effectually exploring the solution space. MOSFO confirms improved optimization by considering both precision and computational efficiency.
The novelty of the ENDDFTL-ABSPFI method is in integrating a CNN-BiLSTM hybrid model for stroke classification with MOSFO for fine-tuning. This incorporation improves the technique’s capability to capture spatial and temporal features while optimizing multiple objectives simultaneously. The approach enables highly accurate and efficient stroke detection, making it adaptive to diverse scenarios and improving overall performance.
Literature works
In11, a Jaccard Residual SqueezeNet is projected to forecast stroke from CT images by incorporating IoT. The Jaccard Residual SqueezeNet combines the Jaccard index in Residual SqueezeNet. Initially, the brain CT image is directed to the base station (BS) utilizing the Fractional Jellyfish Search Pelican Optimizer Algorithm (FJSPOA), and pre-processing is undertaken by the median filter (MF). Moreover, ENet achieves skull segmentation, and feature extraction is performed. Ultimately, Stroke is identified by utilizing the Jaccard Residual SqueezeNet. Phienphanich et al.12 projected the utilization of a facial image database comprising smiling and neutral expressions for categorizing facial weakness, a common indication of a stroke. A facial landmark recognition model separates The faces into right and left sides. An autoencoder technique from a ConvNet decoder and ConvNeXt encoder was developed and used to fine-tune a facial weakness classification method from the projected structure. Raj et al.13 developed a multilevel, multi-modal hierarchical structure for automatic collateral scoring. Notably, a CNN is projected for image choice depending on the visibility of the multi-modal model and collaterals to compare the contralateral and occluded sides of the brain. This method also creates a patient-level forecast by combining automatic ML in the presented structure. Chowdhury et al.14 developed an automated method for identifying the stroke from pre-possessed data employing CNN and other DL approaches. For classification, pre-possessed stroke images are transferred to training and fed into multiple deep structures. Eventually, depending on the classified expression, this method classifies stroke and normal patients. Wu et al.15 projected a computer-assisted DL approach to recognize MLS, focused on predicting conditions in a prognosis-predicting cohort utilizing medical records and brain MLS. Integrated with medical records, patient mortality might be forecast using the multilayer perceptron (MLP) method. Ye et al.16 projected the principles of method integration, algorithm parallelization, and data combination focused on developing an incorporated DL method that depends upon integrating radionics and clinical aspects and examining its application value in prediction forecast. This model implemented the theory of deep combination to the joint examination of several DL approaches. Elhanashi et al.17 developed the labelling and annotation models of the database utilized in DL to classify and detect acute non-stroke in clinical images. This database includes multiple images classified into dual groups: people identified with acute stroke and those without stroke. These models assist in the formation of versatile databases that more precisely mirror real-time situations. In18, forecast techniques depend on non-contrast CT (NCCT) clinical and radiomics aspects are advanced for forecasting the modified Rankin Scale (mRS). Radiomics aspects of the infarct regions are removed from baseline NCCT scans. This paper implemented recursive feature elimination (RFE) and Kruskal–Wallis (KW) tests to choose aspects for developing radionics, fusion, and clinical methods utilizing the support vector machine (SVM) model. Mohamed et al.19 propose a novel probabilistic composite model, integrating Adaptive WavePCA-Autoencoder (AWPA) for pre-processing, Meta-Attention Transformer Autoencoder (MATA) for feature extraction, Genetic Mongoose-Chameleon Optimization (GMCO) for feature selection, and Adaptive Hybrid Exploit Detection Network (AHEDNet) for dynamic ensemble adaptation.
Tejani et al.20 introduce the 2-archive Multi-Objective Cuckoo Search (MOCS2arc) approach to optimize mass and compliance in truss structures and ZDT test functions. Çelik et al.21 present reconfigured single-diode (Reconfig-SDM) and double-diode (Reconfig-DDM) models for more accurate PV cell/module modelling by adding a small resistance in series with the diodes. The squirrel search algorithm (SSA) method is also used for parameter identification. Zhao et al.22 explore the potential of tissue-engineered nerve guidance conduits (NGCs) for peripheral nerve injury (PNI) repair. Kousar et al.23 review DL-based techniques for ischemic stroke prognostication utilizing brain imaging biomarkers, categorize computational approaches for detection, segmentation, and classification, and discuss recent advances and future research directions. Pan et al.24 propose a multi-character classification framework utilizing EEG signals from speech imagery, featuring the wavelet scattering transform (WST) method for feature extraction, Kernel Principal Component Analysis (KPCA) technique for dimensionality reduction, and XGBoost model for classification. Inamdar et al.25 present a dual-stream DL framework for ischemic stroke classification utilizing CT images, featuring a hybrid Dual Attention Mechanism, a Multi-Scale Feature Extraction Module, and an Adaptive Random Vector Functional Link layer for enhanced accuracy, efficiency, and clinical interpretability. Pan et al.26 introduce a novel image reconstruction methodology by using EEG signals, introducing the Deep Visual Representation Model (DVRM) that utilizes a residual-in-residual dense block encoder and deep neural network (DNN) decoder to improve visual stimulus representation accuracy. Prasad, Kumar Saxena, and Laha27 developed a hybrid DL technique for predicting brain cancer occurrence and assessing haemorrhage-related risks utilizing MRI and CT scans, integrating noise removal, refined segmentation, cancer detection with MH-SA-DCNN and Efficient Net, and risk prediction with a Graph-Based DNN and Cox regression model. Du et al.28 examine neurometabolic alterations in the brains of patients with autism spectrum disorders (ASD) using proton magnetic resonance spectroscopy (1H-MRS). Onciul et al.29 explore how AI transforms neuroscience through advanced algorithms, enhancing diagnosis, therapy, and brain-computer interfaces (BCI) while examining challenges and future opportunities for integrating AI with neuroscience. Song et al.30 introduce “TalkingStyle,” a method for creating personalized talking avatars that accurately replicate individuals’ speech and unique talking styles, utilizing separate encoders for style, speech, and motion and a style-conditioned transformer decoder for enhanced control and realism. Talukder et al.31 developed the attention-based convolutional U-Net (ACU-Net) approach for enhanced brain tumour (BT) segmentation in fMRI images, improving accuracy and efficiency by integrating attention mechanisms. Liao et al.32 analyze the onset-to-door time (ODT) in Chinese patients with acute ischemic stroke (AIS) and detect the influencing factors contributing to pre-hospital delays. Dalboni da Rocha et al.33 evaluate AI’s applications, challenges, and potential in pediatric cancer neuroimaging, concentrating on tumour detection, image quality improvement, and the requirement for robust datasets and ethical, explainable models.
Wang et al.34 investigate the role of microglia in social isolation-induced depression utilizing the microglial inhibitor PLX3397, finding that microglial inhibition mitigates depressive-like behaviour in mice by restoring hippocampal function. Tripathi and Kumar35 propose ML techniques for automated BT detection in MRIs, aiming to improve accuracy and reduce the risks associated with conventional biopsy and human examination. Hui et al.36 review recent research on the neuroprotective effects of chinonin, a flavonoid compound from Rhizoma anemarrhena, focusing on its potential to protect against brain injury in neurological diseases. Almansour, Ho, and Palaniappan37 propose an ensemble strategy utilizing the Swin-UNETR technique for improved hypoxic-ischemic encephalopathy (HIE) lesion segmentation in MRI images, illustrating superior performance over U-Net 3D. Song et al.38 introduce a novel method for generating emotionally realistic 3D facial animations synchronized with audio, utilizing the Local-to-global Latent Diffusion Model (LG-LDM) and Emotion-centric Vector Quantized-Variational AutoEncoder (EVQ-VAE) techniques. Du et al.39 propose a low low&high-level BFN distance method and an adaptive multi-label distribution (HBFND-AMLD) method and an adaptive multi-label distribution technique for detecting ASD severity, integrating individual differences in communication and social skills to enhance model accuracy and prevent overfitting-. Luan et al.40 propose a novel DL-based ultrasound localization microscopy (ULM) methodology, AM-Net, with improved localization accuracy, faster processing times, and mitigated computational complexity for microvascular imaging. Song et al.41 present a domain generalization approach that eliminates user-specific calibration, enhancing generalization performance in motor intention classification using EEG data through adaptive weight assignment and multiple decision-making networks. Yu et al.42 propose a DL-based denoising technique, CS-Net, for ULM methodology to improve resolution and mitigate data processing time, enabling real-time clinical imaging. Wang et al.43 explore the clinical application of functional near-infrared spectroscopy (fNIRS) in monitoring disorders of consciousness (DoC), emphasizing its role in diagnosis, BCI, and neuromodulation methods for promoting neurological recovery. Liu et al.44 explore the correlation between the BUN-to-albumin (BUN/Alb) ratio and adverse outcomes in AIS patients utilizing logistic regression (LR) and advanced statistical methods. Krejcar and Namazi45 explore the challenges, methodologies, and clinical potential of multi-scale brain modelling, highlighting its role in advancing scientific understanding, AI applications, and healthcare technologies. Ou et al.46 explore real-time detection of exercise-induced fatigue in seniors by utilizing surface Electromyogram (sEMG) features, specifically kurtosis and skewness, and propose a novel fatigue indicator, Temporal-Mean-Kurtosis (TMK), for low-complexity and sensitive fatigue monitoring in community settings. Huang et al.47 perform a bibliometric analysis of BCI applications in rehabilitation medicine, detecting research trends, key themes, and future potential. Li et al.48 explore using sEMG signals from hand grip for sarcopenia screening in older adults, utilizing ML models for classification and giving interpretability through feature importance estimation utilizing the SHapley Additive explanations (SHAP) model.
The existing studies underscore the crucial progress made in stroke detection, BT classification, and neurological disorder prognosis using DL and AI methods. However, various limitations still exist, such as the reliance on limited datasets, which affects the generalization of models across diverse populations. Many approaches still face difficulty with data imbalance, resulting in biased results. Moreover, the lack of interpretability and explainability in AI models poses challenges for clinical adoption. There is a requirement for robust multi-modal datasets to improve model accuracy and reliability. Ethical concerns around data privacy and patient consent also remain unresolved. Furthermore, integrating clinical and radiometric data for personalized treatment plans remains underexplored. The application of advanced optimization techniques (like GMCO and FJSPOA) is still in the early stages, and their real-world efficacy remains unclear. Research gaps comprise improving model transparency, optimizing feature selection methods, and addressing overfitting through adaptive techniques. Future work should concentrate on multi-institutional collaborations, large-scale clinical trials, and developing explainable AI models for improved clinical integration.
Proposed methodology
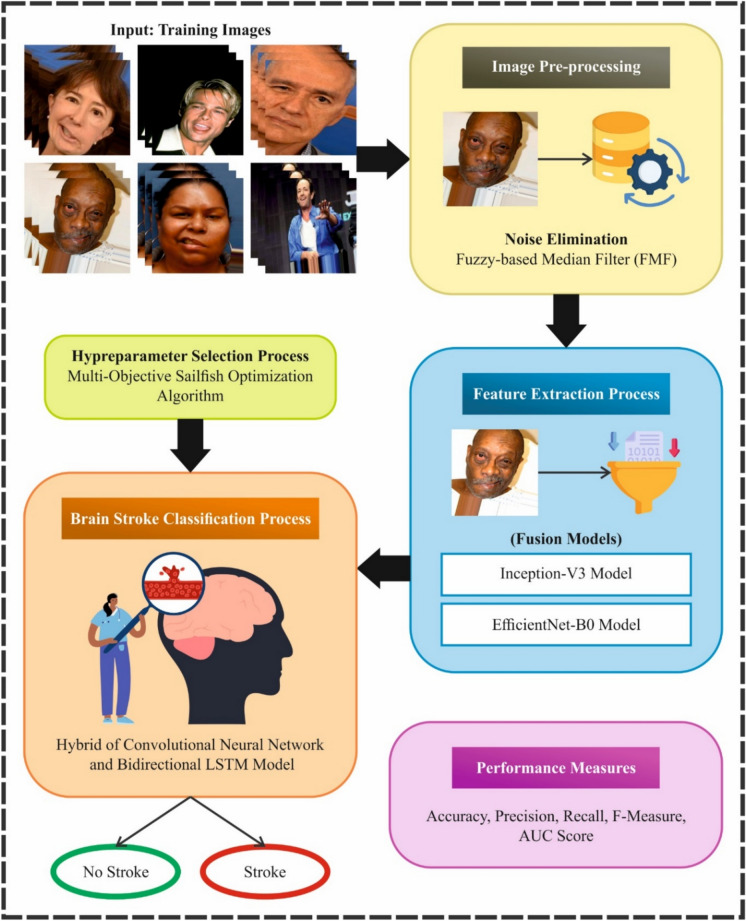
In this manuscript, an ENDDFTL-ABSPFI method is developed. The main intention of the proposed ENDDFTL-ABSPFI technique is to enhance brain stroke detection and classification models using facial imaging. To accomplish that, the ENDDFTL-ABSPFI technique has image pre-processing, fusion of transfer learning, brain stroke prediction using CNN-BiLSTM, and parameter tuning using MOSFO. Figure 1 illustrates the workflow of the ENDDFTL-ABSPFI technique.
Fig. 1.
Workflow of ENDDFTL-ABSPFI method.
Image pre-processing using FMF model
Initially, the image pre-processing stage applies FMF to eliminate the noise in input image data49. This model is chosen due to its superior capacity to mitigate noise while effectively preserving crucial details in brain images. Unlike conventional MF, FMF integrates fuzzy logic (FL) to adaptively adjust the filtering process based on local image characteristics, allowing for enhanced noise reduction in regions with varying levels of detail. This adaptability confirms that significant features, such as edges and contours, are retained while unwanted noise is reduced. Additionally, FMF is computationally efficient, making it appropriate for real-time applications in medical image processing. FMF presents improved edge preservation and smoother results than Gaussian or bilateral filtering techniques. It is particularly valuable in medical imaging, where fine details are crucial for accurate diagnosis.
Utilizing an MF depends on FL to extract noise in the initial phase and recommends an incorporated structure to enhance picture quality. While conventional MF does an admirable job of removing noise, it can distort edges and tiny attributes in clinical pictures. Conversely, the fuzzy-based model permits more adaptability by filtering pixels with diverse membership levels. With this adaptability, edge information might be maintained more advanced while noise might be effectively put down. In this investigation, clinical pictures are filtered utilizing a fuzzy-based MF to five clean and more refined inputs for the succeeding stages of the brain stroke forecast structure. Employing fuzzy-based noise reduction as a method paves the approach for enhanced feature extractor and, eventually, more accurate classification in the predictive methods following stages. During the initial step, the mask is established to identify the pixels  in the image utilizing an FMF analysis as in Eq. (1). The process for FMF denoising is shown below.
in the image utilizing an FMF analysis as in Eq. (1). The process for FMF denoising is shown below.
Step 1: Creating a binary mask Conceal the set 
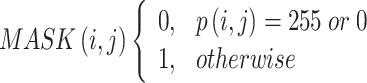 |
1 |
Here,  Is the pixel intensity at the position
Is the pixel intensity at the position  .
.
Step 2: An adaptively filtered window with sizes as in Eqs is selected (2) and (3):
 |
2 |
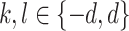 |
3 |
Fusion of transfer learning
Furthermore, fusion models such as Inception-V3 and EfficientNet-B0 perform the feature extraction. This hybrid model is chosen for their capability to capture complex patterns and hierarchical features in brain images effectually. Inception-V3 outperforms in extracting multi-scale features through its inception modules, allowing it to capture fine and coarse details in the images, which is crucial for accurate stroke detection. EfficientNet-B0, on the contrary, is highly effectual, presenting a balanced trade-off between accuracy and computational cost due to its optimized architecture. Together, these models provide a powerful fusion that improves feature extraction by utilizing deep hierarchical learning and computational efficiency. Compared to other models like ResNet or VGG, this integration presents improved performance with fewer parameters, making it ideal for real-time stroke classification tasks in resource-constrained environments. The synergy of these models ensures a robust and scalable feature extraction process.
Inception-V3
In the ImageNet Recognition challenge, a CNN structure known as Inception-v3 was developed50. Google primarily produced it for object detection and image classification. Inception-V3, which combines strategic developments to improve performance, is an evolutionary development of the Inception-V1 structure. More particularly, the original framework is changed to have dual  kernels rather than
kernels rather than 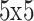 kernels. Additionally, symmetrical convolutions of dimensions
kernels. Additionally, symmetrical convolutions of dimensions  and
and  are applied instead of the
are applied instead of the 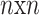 symmetrical convolution in Inception-V1. This model decreases the total parameter counts and generates a deep network, considerably decreasing computational efficiency. Therefore, this development results in enhanced feature extraction performance in the system. As a result, this paper utilizes the Inception-V3 method due to its favourable features. Notably, the design performs better while offering small parameters, which results in more efficient computation processing. This tactical integration is associated with the objectives of this paper. It underlines the importance of maximizing computational complexity without contributing to the system’s capability to remove essential features.
symmetrical convolution in Inception-V1. This model decreases the total parameter counts and generates a deep network, considerably decreasing computational efficiency. Therefore, this development results in enhanced feature extraction performance in the system. As a result, this paper utilizes the Inception-V3 method due to its favourable features. Notably, the design performs better while offering small parameters, which results in more efficient computation processing. This tactical integration is associated with the objectives of this paper. It underlines the importance of maximizing computational complexity without contributing to the system’s capability to remove essential features.
EfficientNet-B0
EfficientNetB0 was developed as a revolutionary network scaling method. They built a fundamental network using a neural structure search and increased it to EfficientNetB0-B7. Multiple coefficients are applied in this model to systematically change the network breadth, depth, and image resolution, as stated in Eq. (4).
 |
 |
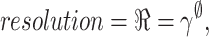 |
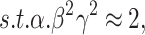 |
 |
4 |
whereas  , and
, and 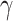 are assessed by grid search and are measured amounts. The specified coefficient indirectly controls the number of extra resources unconcerned with model scaling ∅. Still, the distribution of these added resources to network resolution, depth, and width is defined by the coefficients
are assessed by grid search and are measured amounts. The specified coefficient indirectly controls the number of extra resources unconcerned with model scaling ∅. Still, the distribution of these added resources to network resolution, depth, and width is defined by the coefficients  , and
, and 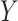 . For all new
. For all new  the equation constraint
the equation constraint  indicates a nearly
indicates a nearly  increase in the total amount of floating point operations per second. This method preserves a balance between precision and efficiency. The increasing popularity of EfficientNet-B0 is due to its ability to strike a balance between size and precision, making the method appropriate for classification and detection tasks. Assuming its capability to preserve this different exchange amongst efficacy and model size, this paper uses EfficientNet-B0, namely small amongst the different EfficientNet methods.
increase in the total amount of floating point operations per second. This method preserves a balance between precision and efficiency. The increasing popularity of EfficientNet-B0 is due to its ability to strike a balance between size and precision, making the method appropriate for classification and detection tasks. Assuming its capability to preserve this different exchange amongst efficacy and model size, this paper uses EfficientNet-B0, namely small amongst the different EfficientNet methods.
Brain stroke prediction using CNN-BiLSTM
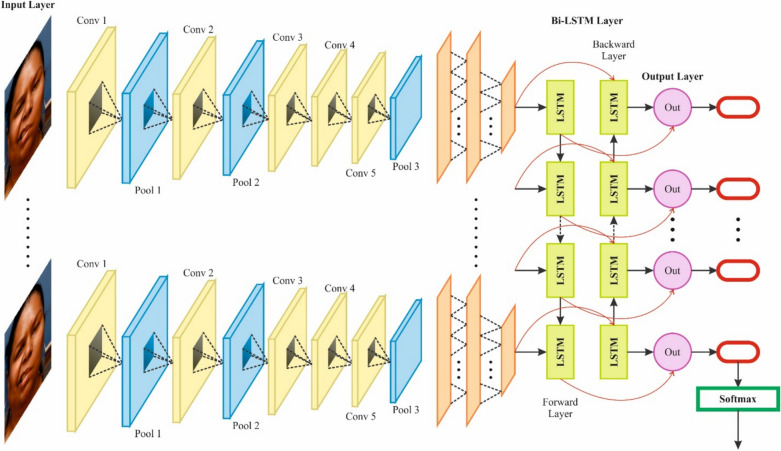
Besides, the hybrid CNN-BiLSTM technique is used for the brain stroke classification model51. This model is chosen for brain stroke prediction because it can effectively handle spatial and temporal dependencies in medical image data. CNNs outperform at extracting spatial features from images, capturing local patterns like edges and textures, which are significant for detecting stroke regions. BiLSTMs, on the contrary, are adept at learning temporal relationships, making them ideal for comprehending sequential or contextual information, which is crucial for tracking stroke progression over time. This integration allows the model to capture detailed image features and the sequential nature of stroke events. Unlike conventional CNNs, which only concentrate on spatial data, the CNN-BiLSTM model enhances stroke detection by integrating spatial context from the images and temporal dynamics. This dual capability presents higher accuracy and robustness in stroke classification compared to other methods that may only address one aspect, such as CNNs or RNNs alone. The model is thus appropriate for handling the complexity of brain stroke prediction. Figure 2 signifies the infrastructure of CNN-BiLSTM.
Fig. 2.
Architecture of CNN-BiLSTM model.
CNNs are usually utilized for processing sequence and image data and are mainly efficient at removing attributes from local areas. During this STFN method, the convolution layer allows the process of learning designs in the information by eliminating local characteristics of the input data. Afterwards, the convolution layer abstracts and removes local information; it is condensed through the pooling layer to decrease computation difficulty and maintain the most significant features. Lastly, the removed attributes are inputted into the bi-directional LSTM layer for additional temporal dependencies modelling. The CNN architecture contains a pooling, input, fully connected (FC), and convolutional layers, finally making the last output. The convolutional layer removes local characteristics from the input data by utilizing filters. In contrast, the pooling layers compress the fea characteristics and maintain essential data, acting as input for the following layers of the bi-directional LSTM.
The fundamental concept of the convolutional process is to implement a sliding window operation on the input information over the convolutional filter (kernel) to remove local characteristics slowly. The primary objective is to take temporal or spatial dependences. Assume that the input data is a 1D sequence  and the convolutional kernel
and the convolutional kernel 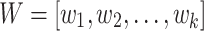 comprises
comprises  parameters, the mathematic formulation of convolution is as shown:
parameters, the mathematic formulation of convolution is as shown:
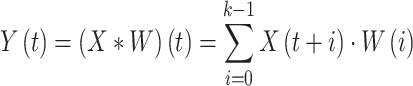 |
5 |
Here, 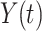 characterizes the
characterizes the  component of the feature mapping after convolution. The convolution kernel
component of the feature mapping after convolution. The convolution kernel  passes over the sequence of the input
passes over the sequence of the input 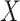 . At all locations; the convolution outcome was gained by multiplying the input information by the equivalent kernel components and summing the products.
. At all locations; the convolution outcome was gained by multiplying the input information by the equivalent kernel components and summing the products.
After the convolutional process, non-linear transformations are typically used over the activation function (like 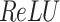 ) to improve the model’s expressive power. The equation for
) to improve the model’s expressive power. The equation for 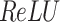 is:
is:
 |
6 |
The activation function of the 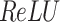 assists the system seizure composite features, prevents the gradient from vanishing, and accelerates the training procedure.
assists the system seizure composite features, prevents the gradient from vanishing, and accelerates the training procedure.
The convolutional layer’s output is usually down-sampled, utilizing a pooling layer to decrease the feature size and computation strength. The equation for m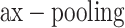 is as shown:
is as shown:
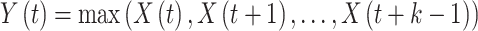 |
7 |
The pooling process minimizes spatial size while protecting the most significant features, which helps alleviate overfitting and enhances the method’s generalizability.
LSTM normally seizures just the forward dependence on time series data, while Bi-LSTM is an expansion of LSTM. Besides utilizing a forward LSTM to handle the input sequence, it uses a reverse LSTM to process the input and the sequence to the beginning. Bi-LSTM can use forward or reverse information to completely seize the context dependences in time-series data. In detail, the Bi-LSTM layer contains dual independent layers of the LSTM: 1) the LSTM of the forward handles data from the sequence start to the end; 2) the LSTM of the forward handles data from the sequence end to the start. The LSTM networking controls the forgetting and storage of data by presenting the input, output, and forget gates. The equations are as shown:
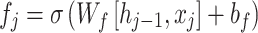 |
8 |
 refers to the forgetting gate’s activation value,
refers to the forgetting gate’s activation value, 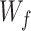 stands for the forgetting gate’s weighted matrix,
stands for the forgetting gate’s weighted matrix, 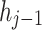 denotes the hidden layer (HL) of the preceding moment,
denotes the hidden layer (HL) of the preceding moment,  represents input at the present moment, and
represents input at the present moment, and  symbolizes the sigmoid activation function.
symbolizes the sigmoid activation function.
The input gate limits the updated ratio of the present input to the state of the cell:
 |
9 |
And the candidate states:
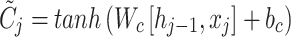 |
10 |
Now, 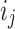 signifies the input gate’s activation value,
signifies the input gate’s activation value,  means the cell’s candidate state. It characterizes the influence of the present input on the cell’s state.
means the cell’s candidate state. It characterizes the influence of the present input on the cell’s state.
The cell state updated equation is as shown:
 |
11 |
This equation establishes that the present cell state contains the preceding state categorized by the gate of forget and the present data designated by the input gate.
The gate of the output defines the last HL as shown:
 |
12 |
The output HL is specified by:
 |
13 |
During this Bi-LSTM, dual LSTM layers handle forward and backward sequence data correspondingly. This multiple-layer architecture might allow all layers to learn more abstract temporal dependency designs.
MOSFO-based parameter tuning
Finally, the MOSFO-based hyperparameter selection process is performed to optimize the classification results of the CNN-BiLSTM method52. This technique is chosen due to its capability to optimize multiple objectives simultaneously, which is significant in complex models like CNN-BiLSTM. MOSFO effectually balances competing objectives such as accuracy, speed, and robustness, ensuring that the model performs well across diverse evaluation metrics. Unlike conventional single-objective optimization techniques, MOSFO can address the trade-offs between various factors, resulting in a more holistic and efficient model. Its swarm-based nature allows it to explore the solution space thoroughly, avoiding local optima and finding a global optimum. This makes MOSFO specifically effectual for fine-tuning DL methods, where multiple hyperparameters must be optimized for maximum performance. By utilizing MOSFO, the parameter tuning process becomes more adaptive and capable of handling complex, non-linear relationships within the model’s parameters, resulting in enhanced stroke prediction outcomes.
The SFO is a bio‐inspired metaheuristic model which draws inspiration from the sailfish’s cooperative searching behaviour. They utilize their agility, speed, and collective tactics to surround and catch prey, normally sardines. The preceding and unique performance of the SFO method reflected only individual objective optimizer issues. The MOSFO proposed in this section lengthens the new SFO to process difficulties with numerous opposed objectives. To do that, the MOSFO model combines external archives and Pareto dominance to process innumerable objectives. The basic processes of the MOSFO model will be presented in the succeeding subsections.
Generally, the multi‐objective optimizer difficulties considered the 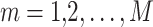 objectives that are concurrently optimized. In real-world settings, optimizer problems need the approval of numerous inequality and equality category constraints. Then, the MOO problem is described as:
objectives that are concurrently optimized. In real-world settings, optimizer problems need the approval of numerous inequality and equality category constraints. Then, the MOO problem is described as:
 |
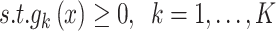 |
 |
14 |
whereas 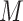 denotes objective counts,
denotes objective counts, 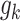 signifies the
signifies the  inequality restriction,
inequality restriction,  refers to an overall number of inequality restrictions,
refers to an overall number of inequality restrictions, 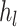 refers to
refers to 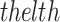 equality constraint, and
equality constraint, and 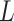 signifies total equality constraint counts. To process the constraints, in the presented MOO structure, all constraints are combined as an added objective to be reduced with the novel target functions. For all inequality constraint
signifies total equality constraint counts. To process the constraints, in the presented MOO structure, all constraints are combined as an added objective to be reduced with the novel target functions. For all inequality constraint  characterizes the decision variable vector and
characterizes the decision variable vector and 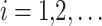 , the function of constraint violation is demonstrated:
, the function of constraint violation is demonstrated:
 |
15 |
These functions 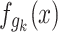 calculate the amount to which candidate solutions
calculate the amount to which candidate solutions 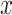 disturb the
disturb the  inequality constraint. As a result, in the event of
inequality constraint. As a result, in the event of 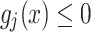 , then
, then  specifies there is no constraint violation.
specifies there is no constraint violation.
In the event of the equivalence constraints 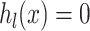 , this is transformed into a reduction of targets by capturing the constraint function’s absolute value based on
, this is transformed into a reduction of targets by capturing the constraint function’s absolute value based on
 |
16 |
Now, the function  determines the absolute divergence from the equality state, guaranteeing that either negative or positive divergence is similar to the violation of the constraint. During this manner, the overview of constraints into the targets, the succeeding multi-target optimizer issue is acquired:
determines the absolute divergence from the equality state, guaranteeing that either negative or positive divergence is similar to the violation of the constraint. During this manner, the overview of constraints into the targets, the succeeding multi-target optimizer issue is acquired:
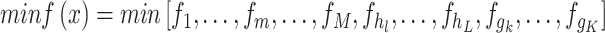 |
17 |
This expression allows the optimizer model to concurrently reduce the unique targets and the functions of constraint violation. This model improves its capacity to navigate complex search areas while possible areas might be fragmented or narrow, finally leading to higher‐quality solutions that satisfy all necessities.
Population initialization
The MOSFO model works with dual significant populations: sardines (prey) and sailfish (predators). To begin the iteration procedure, making the primary sailfish populations and sardines with random locations inside the described searching area was shown:
 |
18 |
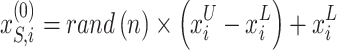 |
19 |
whereas 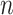 denotes the problem dimension,
denotes the problem dimension, 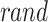 signifies the random generator number in the domain [0, 1], and
signifies the random generator number in the domain [0, 1], and 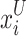 and
and  represents the upper and lower boundaries of the searching region, correspondingly. In the
represents the upper and lower boundaries of the searching region, correspondingly. In the 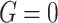 sailfish generation, the ith location is described as the vector.
sailfish generation, the ith location is described as the vector. 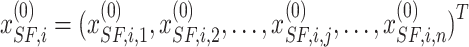 , but the location of the sardines is described as
, but the location of the sardines is described as  . Consequently, the primary sailfish population and sardines are outlined as follows:
. Consequently, the primary sailfish population and sardines are outlined as follows:
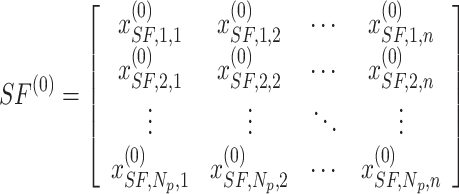 |
20 |
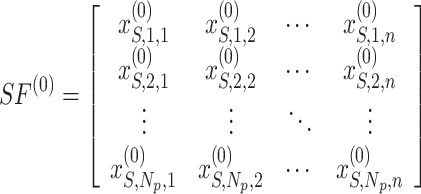 |
21 |
Here,  denotes individual counts within the population.
denotes individual counts within the population.
Archive and elitism management
During MOSFO, an Elitist tactic has been applied to preserve the optimal solutions obtained in the optimizer procedure. The optimal sailfish, 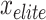 , and the most damaged sardine,
, and the most damaged sardine,  , are conserved through iterations.
, are conserved through iterations.
An outside file is preserved for storing non-dominated solutions. These archives play an essential part in handling the quality and diversity of solutions, guaranteeing a complete estimate of the Pareto front. This archive was upgraded at all iterations with novel non-dominated solutions, and dominated solutions were eliminated.
Attack‐alternation approach
The sailfish upgrade their locations using an alternation approach stimulated by real‐time searching behaviours. During this  generation, the novel location
generation, the novel location  of the
of the  sailfish is computed utilizing the succeeding Eq. (22):
sailfish is computed utilizing the succeeding Eq. (22):
 |
22 |
Now 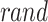
 denotes randomly generated numbers among (0, 1), and
denotes randomly generated numbers among (0, 1), and 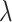 stands for coefficient produced as:
stands for coefficient produced as:
 |
23 |
The 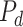 signifies the prey density, demonstrating the prey count at all iterations. As the sailfish gradually search and decrease the population of their prey, the
signifies the prey density, demonstrating the prey count at all iterations. As the sailfish gradually search and decrease the population of their prey, the  parameter is vital in updating the sailfish locations regarding the prey college. Therefore, the adaptable formulations for these parameters are presented as shown:
parameter is vital in updating the sailfish locations regarding the prey college. Therefore, the adaptable formulations for these parameters are presented as shown:
 |
24 |
Meanwhile, 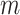 refers to the sailfish count, and
refers to the sailfish count, and  signifies the sardines count. Fitness choice is the major feature that influences the MOSFO approach to performance. The hyperparameter range process considers the solution-encoded system’s proficiency in the candidate solution. The MOSFO technique reflects precision as the primary measure for projecting the FF, which is formulated below.
signifies the sardines count. Fitness choice is the major feature that influences the MOSFO approach to performance. The hyperparameter range process considers the solution-encoded system’s proficiency in the candidate solution. The MOSFO technique reflects precision as the primary measure for projecting the FF, which is formulated below.
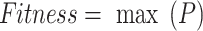 |
25 |
 |
26 |
Meanwhile, 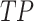 signifies the positive value of true, and
signifies the positive value of true, and 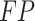 denotes the positive value of false.
denotes the positive value of false.
Performance validation
The experimental validation of the ENDDFTL-ABSPFI technique is studied under the Kaggle dataset53. The dataset contains 2400 images under two classes, stroke and No_stroke, as shown in Table 1. Figure 3 represents the sample images.
Table 1.
Details of dataset.
| Classes | Images |
|---|---|
| Stroke | 1200 |
| No_Stroke | 1200 |
| Total | 2400 |
Fig. 3.
Sample images of (a) no stroke and (b) stroke.
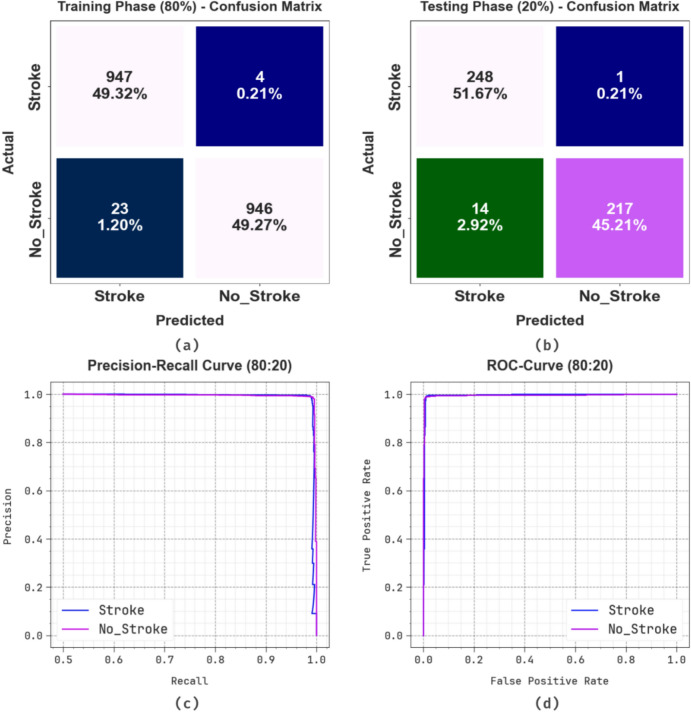
Figure 4 displays the classifier performances of the ENDDFTL-ABSPFI approach below 80%TRPH and 20%TSPH. Figure 4a,b represents the confusion matrices by identifying and classifying all distinct class labels. Figure 4c presents the PR examination, which notified higher performance over all classes. Eventually, Fig. 4d demonstrates the ROC outcome, which signifies capable solutions with great ROC values for discrete classes.
Fig. 4.
80%TRPH and 20%TSPH of (a, b) confusion matrix, (c, d) curve of PR and ROC.
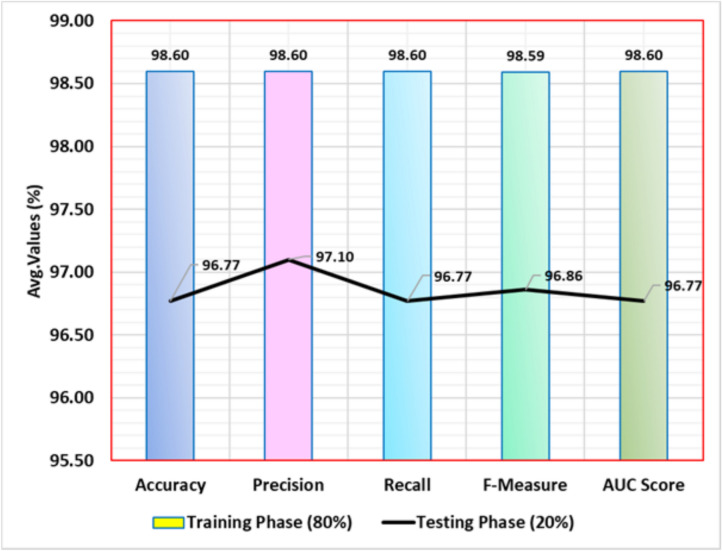
Table 2 and Fig. 5 exemplify the stroke detection of the ENDDFTL-ABSPFI method below 80%TRPH and 20%TSPH. The performances indicate that the ENDDFTL-ABSPFI method accurately recognized the samples. Using 80%TRPH, the ENDDFTL-ABSPFI method delivers average 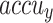 ,
,  ,
,  , and
, and  of 98.60%, 98.60%, 98.60%, 98.59%, and 98.60%, respectively. Additionally, using 20%TSPH, the ENDDFTL-ABSPFI approach provides average
of 98.60%, 98.60%, 98.60%, 98.59%, and 98.60%, respectively. Additionally, using 20%TSPH, the ENDDFTL-ABSPFI approach provides average 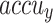 ,
, 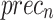 ,
,  , and
, and 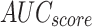 of 96.77%, 97.10%, 96.77%, 96.86%, and 96.77%, respectively.
of 96.77%, 97.10%, 96.77%, 96.86%, and 96.77%, respectively.
Table 2.
Stroke recognition of ENDDFTL-ABSPFI model under 80%TRPH and 20%TSPH.
| Class |  |
 |
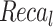 |
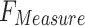 |
 |
|---|---|---|---|---|---|
| TRPH (80%) | |||||
| Stroke | 99.58 | 97.63 | 99.58 | 98.59 | 98.60 |
| No_Stroke | 97.63 | 99.58 | 97.63 | 98.59 | 98.60 |
| Average | 98.60 | 98.60 | 98.60 | 98.59 | 98.60 |
| TSPH (20%) | |||||
| Stroke | 99.60 | 94.66 | 99.60 | 97.06 | 96.77 |
| No_Stroke | 93.94 | 99.54 | 93.94 | 96.66 | 96.77 |
| Average | 96.77 | 97.10 | 96.77 | 96.86 | 96.77 |
Fig. 5.
Average of ENDDFTL-ABSPFI method below 80%TRPH and 20%TSPH.
Figure 6 depicts the training (TRA)  and validation (VAL)
and validation (VAL) 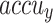 performances of the ENDDFTL-ABSPFI technique under 80%TRPH and 20%TSPH. The values of
performances of the ENDDFTL-ABSPFI technique under 80%TRPH and 20%TSPH. The values of  are computed across a period of 0–50 epochs. The figure underscored that the values of TRA and VAL
are computed across a period of 0–50 epochs. The figure underscored that the values of TRA and VAL 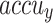 show a growing tendency to indicate the competency of the ENDDFTL-ABSPFI method using maximum performance across numerous repetitions. Moreover, the TRA and VAL
show a growing tendency to indicate the competency of the ENDDFTL-ABSPFI method using maximum performance across numerous repetitions. Moreover, the TRA and VAL 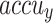 values remain close through the epochs, notifying decreased overfitting and presenting a superior performance of the ENDDFTL-ABSPFI method, guaranteeing reliable calculation on unseen samples.
values remain close through the epochs, notifying decreased overfitting and presenting a superior performance of the ENDDFTL-ABSPFI method, guaranteeing reliable calculation on unseen samples.
Fig. 6.
 curve of ENDDFTL-ABSPFI technique below 80%TRPH and 20%TSPH.
curve of ENDDFTL-ABSPFI technique below 80%TRPH and 20%TSPH.
Figure 7 shows the TRA loss (TRALOS) and VAL loss (VALLOS) graph of the ENDDFTL-ABSPFI approach beneath 80%TRPH and 20%TSPH. The loss values are computed through a period of 0–50 epochs. It is exemplified that the values of TRALOS and VALLOS demonstrate a diminishing trend, which indicates the proficiency of the ENDDFTL-ABSPFI method in harmonizing a trade-off between generality and data fitting. The succeeding dilution in loss and securities values, the ENDDFTL-ABSPFI method’s superior outcome, and tuning the calculation results after a while.
Fig. 7.
Loss curve of ENDDFTL-ABSPFI technique under 80%TRPH and 20%TSPH.
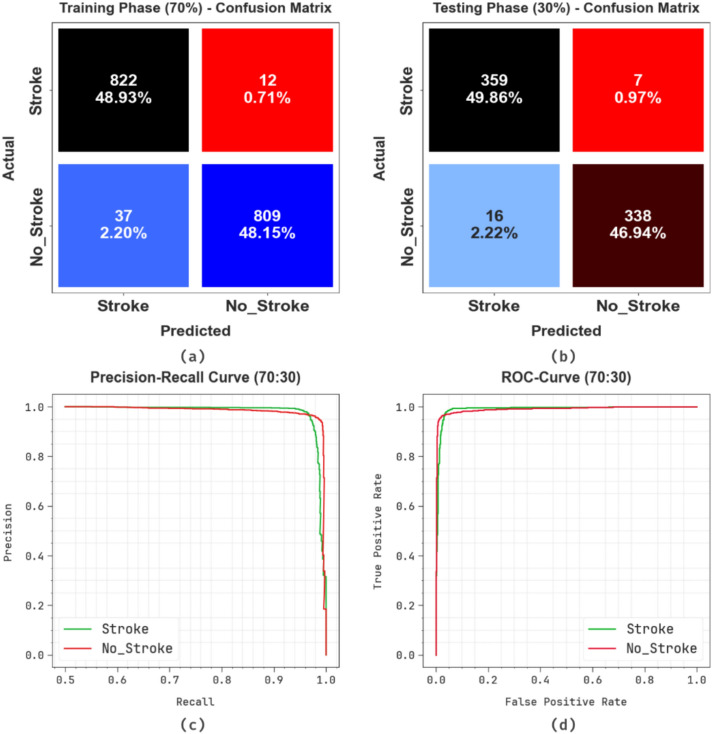
Figure 8 shows the classifier performances of the ENDDFTL-ABSPFI model below 70%TRPH and 30%TSPH. Figure 8a,b reveals the confusion matrices through specific classification and identification of all different classes. Figure 8c presents the PR outcome, which notified enhanced performance through all classes. Finally, Fig. 8d signifies the ROC study, which illustrates skilful solutions with great ROC values for dissimilar classes.
Fig. 8.
70%TRPH and 30%TSPH of (a, b) confusion matrix, (c, d) curve of PR and ROC.
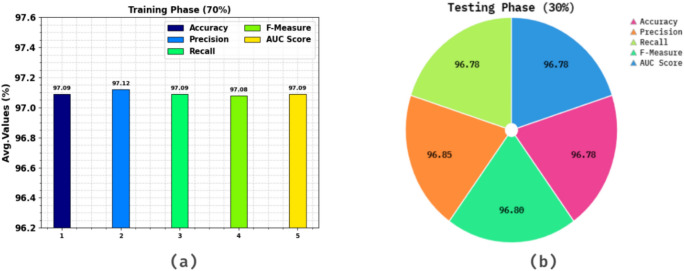
Table 3 and Fig. 9 demonstrate the stroke detection of ENDDFTL-ABSPFI methodology below 70%TRPH and 30%TSPH. The performances indicate that the ENDDFTL-ABSPFI methodology accurately recognized the samples. Using 70%TRPH, the ENDDFTL-ABSPFI methodology provides average 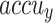 ,
, 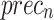 ,
,  , and
, and 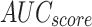 of 97.09%, 97.12%, 97.09%, 97.08%, and 97.09%, respectively. Moreover, using 30%TSPH, the ENDDFTL-ABSPFI approach delivers average
of 97.09%, 97.12%, 97.09%, 97.08%, and 97.09%, respectively. Moreover, using 30%TSPH, the ENDDFTL-ABSPFI approach delivers average  ,
,  ,
,  , and
, and  of 97.09%, 97.12%, 97.09%, 97.08%, and 97.09%, correspondingly.
of 97.09%, 97.12%, 97.09%, 97.08%, and 97.09%, correspondingly.
Table 3.
Stroke recognition of ENDDFTL-ABSPFI model below 70%TRPH and 30%TSPH.
| Class |  |
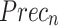 |
 |
 |
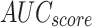 |
|---|---|---|---|---|---|
| TRPH (70%) | |||||
| Stroke | 98.56 | 95.69 | 98.56 | 97.11 | 97.09 |
| No_Stroke | 95.63 | 98.54 | 95.63 | 97.06 | 97.09 |
| Average | 97.09 | 97.12 | 97.09 | 97.08 | 97.09 |
| TSPH (30%) | |||||
| Stroke | 98.09 | 95.73 | 98.09 | 96.90 | 96.78 |
| No_Stroke | 95.48 | 97.97 | 95.48 | 96.71 | 96.78 |
| Average | 96.78 | 96.85 | 96.78 | 96.80 | 96.78 |
Fig. 9.
Average of ENDDFTL-ABSPFI model below 70%TRPH and 30%TSPH.
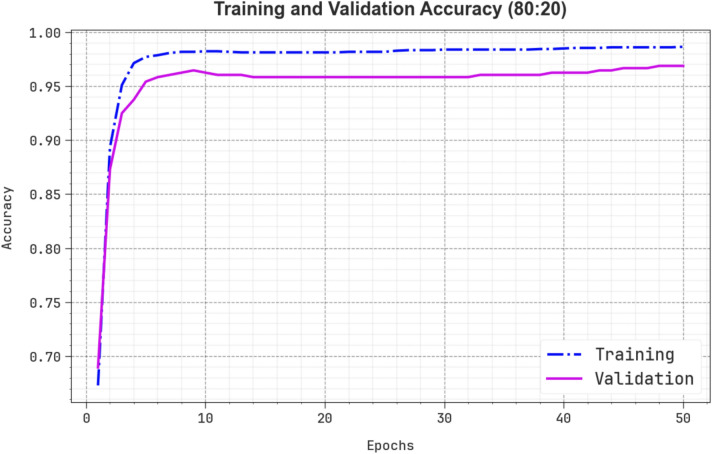
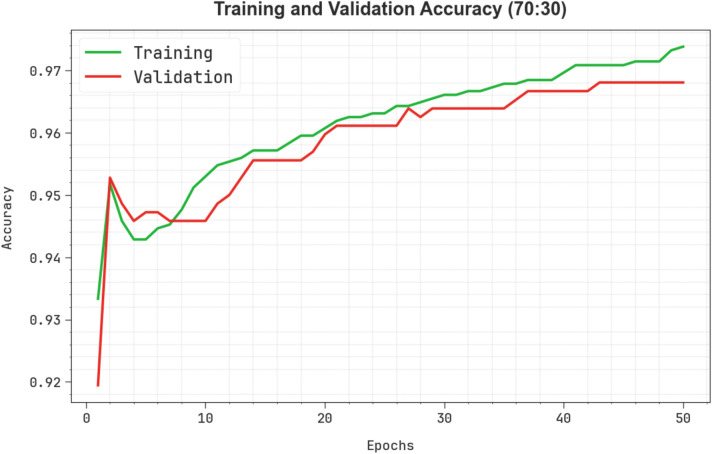
Figure 10 showcases the TRA  and VAL
and VAL  performances of the ENDDFTL-ABSPFI method below 70%TRPH and 30%TSPH. The values of
performances of the ENDDFTL-ABSPFI method below 70%TRPH and 30%TSPH. The values of 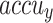 are computed across a period of 0–50 epochs. The figure underscored that the TRA and VAL
are computed across a period of 0–50 epochs. The figure underscored that the TRA and VAL 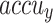 values present a cumulative tendency, indicating the capacity of the ENDDFTL-ABSPFI approach with maximum outcome through multiple repetitions. In addition, the TRA and VAL
values present a cumulative tendency, indicating the capacity of the ENDDFTL-ABSPFI approach with maximum outcome through multiple repetitions. In addition, the TRA and VAL 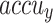 values remain close across the epochs, notifying lesser overfitting and expressing superior outcomes of the ENDDFTL-ABSPFI approach, assuring steady calculation on unseen samples.
values remain close across the epochs, notifying lesser overfitting and expressing superior outcomes of the ENDDFTL-ABSPFI approach, assuring steady calculation on unseen samples.
Fig. 10.
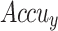 curve of ENDDFTL-ABSPFI model below 70%TRPH and 30%TSPH.
curve of ENDDFTL-ABSPFI model below 70%TRPH and 30%TSPH.
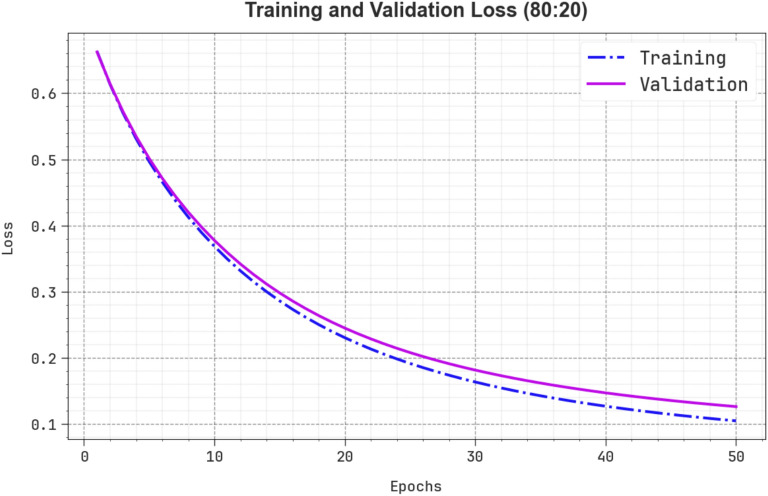
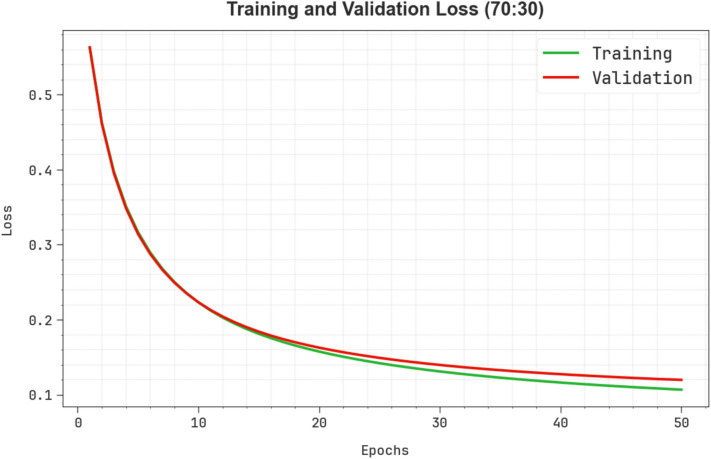
Figure 11 shows the TRALOS and VALLOS graph of the ENDDFTL-ABSPFI approach below 70%TRPH and 30%TSPH. The loss values are computed throughout 0–50 epochs. The values of TRALOS and VALLOS represent a declining tendency, which indicates the proficiency of the ENDDFTL-ABSPFI method in equalizing a trade-off between simplification and data fitting. The successive dilution in loss and securities values increased the outcome of the ENDDFTL-ABSPFI method and tuned the calculation results gradually.
Fig. 11.
Loss curve of ENDDFTL-ABSPFI model below 70%TRPH and 30%TSPH.
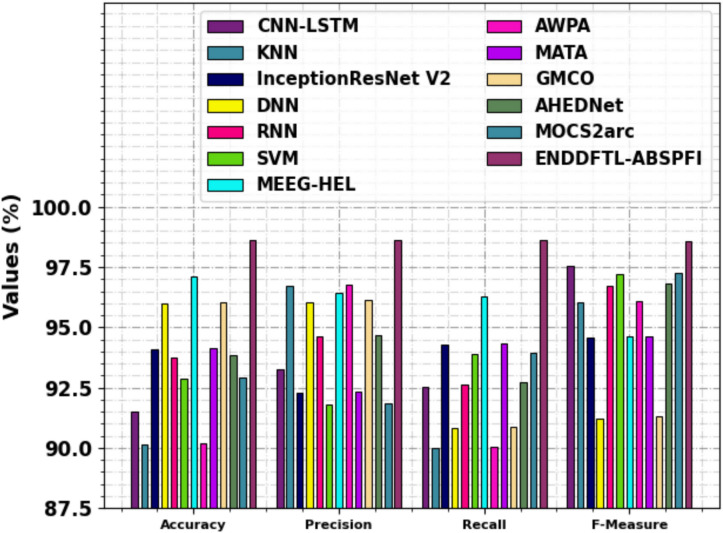
Table 4 and Fig. 12 study the comparison analysis of the ENDDFTL-ABSPFI approach through the existing methodologies19,20,54–56. The performances underscored that the CNN-LSTM, KNN, InceptionResNet V2, DNN, RNN, and SVM techniques have stated poorer performance. Likewise, the MEEG-HEL, AWPA, MATA, GMCO, AHEDNet, and MOCS2arc methods gained closer solutions. In addition, the ENDDFTL-ABSPFI methodology indicated maximum performance with increased  ,
, 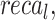 and
and 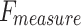 of 98.60%, 98.60%, 98.60%, and 98.59%, respectively.
of 98.60%, 98.60%, 98.60%, and 98.59%, respectively.
Table 4.
Comparative study of ENDDFTL-ABSPFI methodology with existing models.
| Methdology |  |
 |
 |
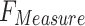 |
|---|---|---|---|---|
| CNN-LSTM | 91.50 | 93.26 | 92.54 | 97.57 |
| KNN | 90.16 | 96.71 | 90.00 | 96.03 |
| InceptionResNet V2 | 94.09 | 92.27 | 94.29 | 94.57 |
| DNN | 95.98 | 96.06 | 90.82 | 91.24 |
| RNN | 93.75 | 94.62 | 92.64 | 96.73 |
| SVM | 92.87 | 91.79 | 93.88 | 97.22 |
| MEEG-HEL | 97.10 | 96.42 | 96.27 | 94.61 |
| AWPA | 90.21 | 96.77 | 90.05 | 96.09 |
| MATA | 94.16 | 92.32 | 94.35 | 94.62 |
| GMCO | 96.05 | 96.12 | 90.88 | 91.30 |
| AHEDNet | 93.83 | 94.69 | 92.71 | 96.80 |
| MOCS2arc | 92.93 | 91.85 | 93.94 | 97.28 |
| ENDDFTL-ABSPFI | 98.60 | 98.60 | 98.60 | 98.59 |
Fig. 12.
Comparative analysis of ENDDFTL-ABSPFI methodology with existing models.
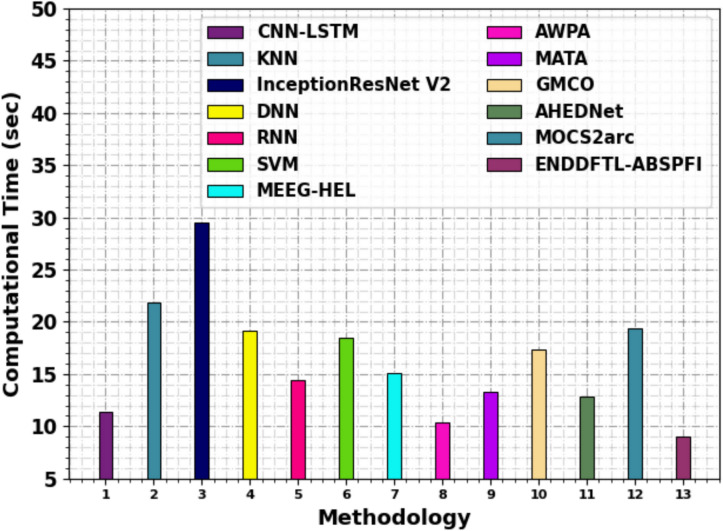
Table 5 and Fig. 13 illustrates the computational time (CT) analysis of the ENDDFTL-ABSPFI technique with existing methods. Among the listed models, the ENDDFTL-ABSPFI model depicts the shortest CT at 9.07 s, followed by AWPA at 10.38 s. The CNN-LSTM model has a relatively moderate time of 11.34 s, while models like AHEDNet at 12.80 s, MATA at 13.32 s, and RNN at 14.43 s exhibit slightly higher CTs. InceptionResNet V2 and MOCS2arc portrys the longest CT at 29.54 s and 19.34 s, respectively. Other models such as KNN, DNN, SVM, MEEG-HEL, and GMCO have varying CT between 15 to 21 s, indicating the trade-off between accuracy and computational efficiency in the models tested.
Table 5.
CT analysis of ENDDFTL-ABSPFI approach with existing methods.
| Methodology | CT (sec) |
|---|---|
| CNN-LSTM | 11.34 |
| KNN | 21.91 |
| InceptionResNet V2 | 29.54 |
| DNN | 19.10 |
| RNN | 14.43 |
| SVM | 18.53 |
| MEEG-HEL | 15.13 |
| AWPA | 10.38 |
| MATA | 13.32 |
| GMCO | 17.30 |
| AHEDNet | 12.80 |
| MOCS2arc | 19.34 |
| ENDDFTL-ABSPFI | 9.07 |
Fig. 13.
CT analysis of ENDDFTL-ABSPFI approach with existing methods.
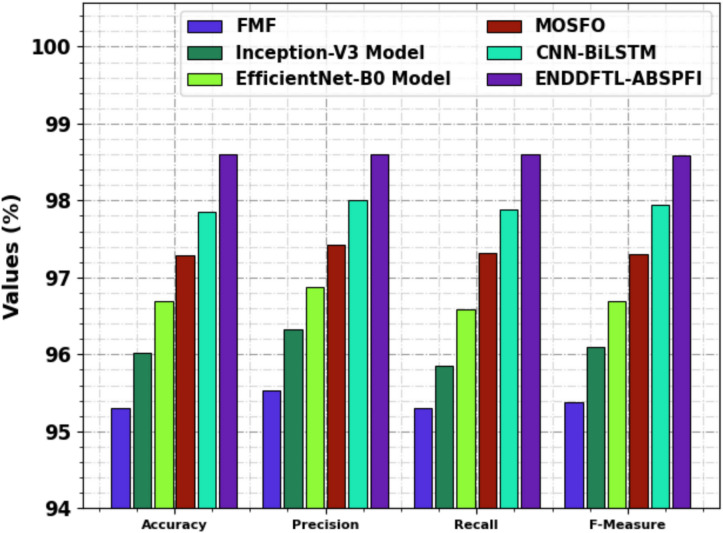
The ablation study of the ENDDFTL-ABSPFI technique is demonstrated in Table 6 and Fig. 14. The table showcases the performance metrics of various techniques based on  ,
, 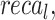 and
and  . The ENDDFTL-ABSPFI technique outperforms all others with an
. The ENDDFTL-ABSPFI technique outperforms all others with an  ,
, 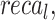 and
and  of 98.60%, 98.60%, 98.60%, and 98.59%, respectively. Following closely, the CNN-BiLSTM model achieves an
of 98.60%, 98.60%, 98.60%, and 98.59%, respectively. Following closely, the CNN-BiLSTM model achieves an 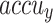 of 97.86%,
of 97.86%, 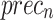 of 98.01%,
of 98.01%, 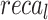 of 97.89%, and
of 97.89%, and 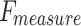 of 97.95%. MOSFO illustrates slightly lower results with an
of 97.95%. MOSFO illustrates slightly lower results with an 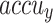 of 97.29%,
of 97.29%, 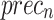 of 97.42%,
of 97.42%, 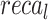 of 97.32%, and
of 97.32%, and  of 97.31%. The EfficientNet-B0 model achieves an
of 97.31%. The EfficientNet-B0 model achieves an  of 96.70%,
of 96.70%, 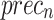 of 96.87%,
of 96.87%,  of 96.59%, and
of 96.59%, and 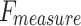 of 96.70%, while Inception-V3 achieves
of 96.70%, while Inception-V3 achieves  of 96.02%,
of 96.02%, 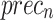 of 96.32%,
of 96.32%,  of 95.86%, and
of 95.86%, and 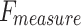 of 96.09%. The FMF technique performs the least with an
of 96.09%. The FMF technique performs the least with an  of 95.30%,
of 95.30%, 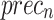 of 95.53%,
of 95.53%, 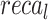 of 95.31%, and
of 95.31%, and 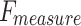 of 95.38%. The ENDDFTL-ABSPFI technique outperforms others, showing robust outcomes.
of 95.38%. The ENDDFTL-ABSPFI technique outperforms others, showing robust outcomes.
Table 6.
Result analysis of the ablation study of ENDDFTL-ABSPFI technique.
| Technique |  |
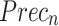 |
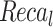 |
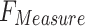 |
|---|---|---|---|---|
| FMF | 95.30 | 95.53 | 95.31 | 95.38 |
| Inception-V3 model | 96.02 | 96.32 | 95.86 | 96.09 |
| EfficientNet-B0 model | 96.70 | 96.87 | 96.59 | 96.70 |
| MOSFO | 97.29 | 97.42 | 97.32 | 97.31 |
| CNN-BiLSTM | 97.86 | 98.01 | 97.89 | 97.95 |
| ENDDFTL-ABSPFI | 98.60 | 98.60 | 98.60 | 98.59 |
Fig. 14.
Result analysis of the ablation study of ENDDFTL-ABSPFI technique.
Conclusion
In this study, the ENDDFTL-ABSPFI method is developed. The main intention of the proposed ENDDFTL-ABSPFI technique is to enhance the detection and classification model of brain stroke using facial imaging. Initially, the image pre-processing stage applies FMF to eliminate the noise in input image data. Furthermore, fusion models such as Inception-V3 and EfficientNet-B0 executed the feature extraction process. Besides, the hybrid CNN-BiLSTM technique is used for the brain stroke classification model. At last, the MOSFO model-based hyperparameter selection process is carried out to optimize the classification outcomes of the CNN-BiLSTM technique. The simulation validation of the ENDDFTL-ABSPFI technique is investigated under the Kaggle dataset concerning various measures. The comparative evaluation of the ENDDFTL-ABSPFI technique portrayed a superior accuracy value of 98.60% over existing methods. There are several limitations to the ENDDFTL-ABSPFI technique. First, relying on a single dataset may limit the model’s generalizability to diverse patient populations and imaging conditions. Second, the performance of the method might be affected by the quality of input images, particularly in the presence of noise or artefacts that were not thoroughly addressed in all real-world scenarios. Additionally, the computational demands of the model could affect its deployment in low-resource settings where processing power is limited. The lack of interpretability in some ML models also challenges clinical adoption, as healthcare professionals require transparent decision-making processes. Future work should consider validating the model across more diverse datasets, improving its robustness to image variability, and exploring techniques for improving model explainability. Further research can also mitigate computational complexity and adapt the model for real-time applications in clinical environments.
Acknowledgements
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number RGP2/271/46. Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R77), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Deanship of Scientific Research at Northern Border University, Arar, KSA for funding this research work through the project number NBU-FFR-2025- 2847-03. The authors are thankful to the Deanship of Graduate Studies and Scientific Research at University of Bisha for supporting this work through the Fast-Track Research Support Program. This study is supported via funding from Prince Sattam bin Abdulaziz University project number (PSAU/2025/R/1446).
Author contributions
Fadwa Alrowais: Conceptualization, methodology development, experiment, formal analysis, investigation, writing. Mohammed Alqahtani: Formal analysis, investigation, validation, visualization, writing. Jahangir khan: Formal analysis, review and editing. Da’ad Albalawneh : Methodology, investigation. Abdulwhab Alkharashi: Review and editing. Samah Al Zanin: Discussion, review and editing. Radwa Marzouk: Discussion, review and editing. Achraf Ben Miled: Conceptualization, methodology development, investigation, supervision, review and editing. All authors have read and agreed to the published version of the manuscript.
Data availability
The data supporting this study’s findings are openly available in the Kaggle repository at https://www.kaggle.com/datasets/danish003/face-images-of-acute-stroke-and-non-acute-stroke, reference number53.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sirsat, M. S., Fermé, E. & Camara, J. Machine learning for brain stroke: A review. J. Stroke Cerebrovasc. Dis.29(10), 105162 (2020). [DOI] [PubMed] [Google Scholar]
- 2.AL-Nuaimi, B. T., Suhail, R. A. & El-kenawy, E. S. M. Adaptive feature selection based on machine learning algorithms for lung tumors diagnosis and the COVID-19 index. J. Intell. Syst. Internet Things11(2), 24 (2024). [Google Scholar]
- 3.Islam, M. S., Hussain, I., Rahman, M. M., Park, S. J. & Hossain, M. A. Explainable artificial intelligence model for stroke prediction using EEG signal. Sensors22(24), 9859 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hilbert, A. et al. Data-efficient deep learning of radiological image data for outcome prediction after endovascular treatment of patients with acute ischemic stroke. Comput. Biol. Med.115, 103516 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Choi, Y. A. et al. Deep learning-based stroke disease prediction system using real-time bio signals. Sensors21(13), 4269 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur, M., Sakhare, S. R., Wanjale, K. & Akter, F. [Retracted] Early stroke prediction methods for prevention of strokes. Behav. Neurol.2022(1), 7725597 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Mouridsen, K., Thurner, P. & Zaharchuk, G. Artificial intelligence applications in stroke. Stroke51(8), 2573–2579 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Gautam, A. & Raman, B. Towards effective classification of brain hemorrhagic and ischemic stroke using CNN. Biomed. Signal Process. Control63, 102178 (2021). [Google Scholar]
- 9.Wang, Y. et al. The Third China National Stroke Registry (CNSR-III) for patients with acute ischaemic stroke or transient ischaemic attack: Design, rationale and baseline patient characteristics. Stroke Vasc. Neurol.4(3), 24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kavitha, P., Shini, R. S. & Priya, R. An implementation of statistical feature algorithms for the detection of brain tumor. Full Length Article1(2), 57–62 (2022). [Google Scholar]
- 11.Sreekumari, A. B. & Yesudasan Paulsy, A. T. Hybrid deep learning based stroke detection using CT images with routing in an IoT environment. Netw. Comput. Neural Syst.8, 1–40 (2025). [DOI] [PubMed] [Google Scholar]
- 12.Phienphanich, P. et al. Generalizing a small facial image dataset using facial generative adversarial networks for Stroke’s facial weakness screening. IEEE Access11, 64886–64896 (2023). [Google Scholar]
- 13.Raj, R., Pruthviraja, D., Gupta, A., Mathew, J., Kannath, S. K., Prakash, A. & Rajan, J. Multilevel multi-modal framework for automatic collateral scoring in brain stroke. IEEE Access (2024).
- 14.Chowdhury, N. A., Mahmud, T., Barua, A., Basnin, N., Barua, K., Iqbal, A., Hossain, M. S., Andersson, K., Kaiser, M. S., Hossain, M. S. & Das, S. A novel approach to detect stroke from 2d images using deep learning. In International Conference on Big Data, IoT and Machine Learning 239–253. (Springer, 2023).
- 15.Wu, A. R., Hsieh, S. Y., Chou, H. H., Lai, C. S., Hung, J. Y., Wang, B. & Tsai, Y. S. Deep learning-based prediction of mortality using brain midline shift and clinical information. Heliyon (2025). [DOI] [PMC free article] [PubMed]
- 16.Ye, W. et al. OEDL: An optimized ensemble deep learning method for predicting acute ischemic stroke prognoses using union features. Front. Neurol.14, 1158555 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elhanashi, A., Saponara, S. & Zheng, Q. Annotation facial images for stroke classification acute vs non acute. In 2024 Sixth International Conference on Intelligent Computing in Data Sciences (ICDS) 1–7. (IEEE, 2024).
- 18.Zhang, L. et al. Non-contrast CT radiomics and machine learning for outcomes prediction of patients with acute ischemic stroke receiving conventional treatment. Eur. J. Radiol.165, 110959 (2023). [DOI] [PubMed] [Google Scholar]
- 19.Mohamed, A. A., Al-Saleh, A., Sharma, S. K. & Tejani, G. G. Zero-day exploits detection with adaptive WavePCA-autoencoder (AWPA) adaptive hybrid exploit detection network (AHEDNet). Sci. Rep.15(1), 4036 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tejani, G. G., Mashru, N., Patel, P., Sharma, S. K. & Celik, E. Application of the 2-archive multi-objective cuckoo search algorithm for structure optimization. Sci. Rep.14(1), 31553 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Çelik, E. et al. Reconfigured single-and double-diode models for improved modelling of solar cells/modules. Sci. Rep.15(1), 2101 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao, Y. et al. Peripheral nerve injury repair by electrical stimulation combined with graphene-based scaffolds. Front. Bioeng. Biotechnol.12, 1345163 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kousar, T., Rahim, M. S. M., Iqbal, S., Yousaf, F. & Sanaullah, M. Applications of deep learning algorithms in ischemic stroke detection, segmentation, and classification. Artif. Intell. Rev.58(5), 1–48 (2025). [Google Scholar]
- 24.Pan, H., Wang, Y., Li, Z., Chu, X., Teng, B. & Gao, H. A complete scheme for multi-character classification using EEG signals from speech imagery. IEEE Trans. Biomed. Eng. (2024). [DOI] [PubMed]
- 25.Inamdar, M. A., Gudigar, A., Raghavendra, U., Salvi, M., Aman, R. R. A. B. R., Gowdh, N. F. M., Ahir, I. A. B. M., Kamaruddin, M. S. B., Kadir, K. A. A., Molinari, F. & Hegde, A. A Dual-stream deep learning architecture with adaptive random vector functional link for multi-center ischemic stroke classification. IEEE Access. (2025).
- 26.Pan, H., Li, Z., Fu, Y., Qin, X. & Hu, J. Reconstructing visual stimulus representation from EEG signals based on deep visual representation model. IEEE Trans. Hum. Mach. Syst. (2024)
- 27.Prasad, R., Kumar Saxena, A. & Laha, S. Prediction of brain cancer occurrence and risk assessment of brain hemorrhage using hybrid deep learning technique. Cancer Invest.43(1), 80–102 (2025). [DOI] [PubMed] [Google Scholar]
- 28.Du, Y. et al. Neurometabolite levels in the brains of patients with autism spectrum disorders: A meta-analysis of proton magnetic resonance spectroscopy studies (N = 1501). Mol. Psychiatry28(7), 3092–3103 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Onciul, R. et al. Artificial intelligence and neuroscience: Transformative synergies in brain research and clinical applications. J. Clin. Med.14(2), 550 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song, W., Wang, X., Zheng, S., Li, S., Hao, A. & Hou, X. TalkingStyle: Personalized speech-driven 3D facial animation with style preservation. IEEE Trans. Visual. Comput. Graph. (2024). [DOI] [PubMed]
- 31.Talukder, M. A. et al. ACU-Net: Attention-based convolutional U-Net model for segmenting brain tumors in fMRI images. Digit. Health11, 20552076251320290 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao, Y. et al. Analysis of onset-to-door time and its influencing factors in Chinese patients with acute ischemic stroke during the 2020 COVID-19 epidemic: A preliminary, prospective, multicenter study. BMC Health Serv. Res.24(1), 615 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalboni da Rocha, J. L. et al. Artificial intelligence for neuroimaging in pediatric cancer. Cancers17(4), 622 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, L. et al. Pexidartinib (PLX3397) through restoring hippocampal synaptic plasticity ameliorates social isolation-induced mood disorders. Int. Immunopharmacol.113, 109436 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tripathi, M. N. & Kumar, A. Brain tumor diagnosis using image classifier. In Artificial Intelligence in Biomedical and Modern Healthcare Informatics 513–524. (Academic Press, 2025).
- 36.Hui, Z. et al. Mechanisms and therapeutic potential of chinonin in nervous system diseases. J. Asian Nat. Prod. Res.26(12), 1405–1420 (2024). [DOI] [PubMed] [Google Scholar]
- 37.Almansour, M., Ho, M. L. & Palaniappan, K. Enhancing lesion segmentation in the BONBID-HIE challenge: An ensemble strategy. In AI for Brain Lesion Detection and Trauma Video Action Recognition: First BONBID-HIE Lesion Segmentation Challenge and First Trauma Thompson Challenge, Held in Conjunction with MICCAI 2023, Vancouver, BC, Canada, October 16 and 12, 2023, Proceedings, 14567, 14 (2025).
- 38.Song, W., Wang, X., Jiang, Y., Li, S., Hao, A., Hou, X. & Qin, H. Expressive 3d facial animation generation based on local-to-global latent diffusion. IEEE Trans. Visual. Comput. Graph. (2024). [DOI] [PubMed]
- 39.Du, J., Wang, S., Chen, R. & Wang, S. Improving fMRI-based autism severity identification via brain network distance and adaptive label distribution learning. IEEE Trans. Neural Syst. Rehabil. Eng.33, 162–174 (2025). [DOI] [PubMed] [Google Scholar]
- 40.Luan, S. et al. Deep learning for fast super-resolution ultrasound microvessel imaging. Phys. Med. Biol.68(24), 245023 (2023). [DOI] [PubMed] [Google Scholar]
- 41.Song, H. et al. Domain generalization through latent distribution exploration for motor imagery EEG classification. Neurocomputing614, 128889 (2025). [Google Scholar]
- 42.Yu, X. et al. Deep learning for fast denoising filtering in ultrasound localization microscopy. Phys. Med. Biol.68(20), 205002 (2023). [DOI] [PubMed] [Google Scholar]
- 43.Wang, N. et al. Functional near-infrared spectroscopy for the assessment and treatment of patients with disorders of consciousness. Front. Neurol.16, 1524806 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu, H. et al. The interrelation of blood urea nitrogen-to-albumin ratio with three-month clinical outcomes in acute ischemic stroke cases: A secondary analytical exploration derived from a prospective cohort study. Int. J. Gener. Med.8, 5333–5347 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krejcar, O. & Namazi, H. Multi-scale brain modeling: Bridging microscopic and macroscopic brain dynamics for clinical and technological applications. Front. Cell. Neurosci.19, 1537462 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ou, J. et al. Detecting muscle fatigue among community-dwelling senior adults with shape features of the probability density function of sEMG. J. Neuroeng. Rehabil.21(1), 196 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang, J., Huang, L., Li, Y. & Fang, F. A bibliometric analysis of the application of brain-computer interface in rehabilitation medicine over the past 20 years. J. Multidiscip. Healthc. 1297–1317 (2025). [DOI] [PMC free article] [PubMed]
- 48.Li, N. et al. Exploration of a machine learning approach for diagnosing sarcopenia among Chinese community-dwelling older adults using sEMG-based data. J. Neuroeng. Rehabil.21(1), 69 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanagalakshmi, K. & Nisha, H. B. Lung cancer prediction with improved graph convolutional neural networks. Available at SSRN 5069314.
- 50.Khan, T., Yasir, M. & Choi, C. Attention-enhanced optimized deep ensemble network for effective facial emotion recognition. Alex. Eng. J.119, 111–123 (2025). [Google Scholar]
- 51.Chen, H., Liu, H. & Yang, W. STFNIoT: Lightweight IoT intrusion detection based on explainable analysis using spatiotemporal fusion networks (2025).
- 52.Sedak, M., Rosić Vitas, M. & Rosić, B. Multi-objective hybrid sailfish optimization algorithm for planetary gearbox and mechanical engineering design optimization problems. Comput. Model. Eng. Sci. (2025).
- 53.https://www.kaggle.com/datasets/danish003/face-images-of-acute-stroke-and-non-acute-stroke
- 54.Ali, M. et al. Classification of physiotherapy exercise of stroke patients using deep transfer learning and fuzzy logic: A novel approach. Ain Shams Eng. J.15(10), 102940 (2024). [Google Scholar]
- 55.Saleem, M. A., Javeed, A., Akarathanawat, W., Chutinet, A., Suwanwela, N. C., Asdornwised, W., Chaitusaney, S., Deelertpaiboon, S., Srisiri, W., Benjapolakul, W. & Kaewplung, P. Innovations in stroke identification: A machine learning-based diagnostic model using neuroimages. IEEE Access (2024).
- 56.Lee, M., Park, H. Y., Park, W., Kim, K. T., Kim, Y. H. & Jeong, J. H. Multi-task heterogeneous ensemble learning-based cross-subject EEG classification under stroke patients. IEEE Trans. Neural Syst. Rehabil. Eng. (2024). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study’s findings are openly available in the Kaggle repository at https://www.kaggle.com/datasets/danish003/face-images-of-acute-stroke-and-non-acute-stroke, reference number53.