Abstract
Lack of sleep is a common problem in current society, which can induce various brain dysfunctions. Neuroinflammation is a typical reaction caused by sleep deficit and is considered as a common basis for various neurological disorders and cognitive impairments, but the related mechanisms have not been fully clarified. The circadian clock protein plays a critical role in maintaining physiological homeostasis, including sleep/wake cycles. Circadian disorders induced by sleep deficit might contribute to the development of neuroinflammation. In the current study, we observed that sleep deprivation (SD) induced elevated expression of High-mobility group box 1 (HMGB1), one of the most important mediators of neuroinflammation, in the cortical microglia and cerebrospinal fluids. Moreover, acetylation-dependent nuclear export of HMGB1 was involved in up-regulation and secretion of HMGB1 after sleep deprivation. Further studies indicated that sleep deprivation induced an increase in the expression of acetyltransferase p300 and a decrease in the expression of deacetylase SIRT1, which synergistically enhanced the acetylation level of HMGB1 in the cortical microglial cells, thereby triggered the nuclear export and secretion of HMGB1. Most importantly, circadian clock protein PER2 constitutively interacted with p300 and inhibited its expression in the microglial cells, which can be interrupted by PER2 downregulation upon sleep deprivation, leading to the increased expression of p300 and acetylation and secretion of HMGB1. The truncated PER2 mutant without p300 binding ability lost its ability to regulate p300 expression, indicating that PER2 functioned as a co-suppressor of p300 in regulating acetylation and expression of HMGB1. Taken together, data in this study reveal a new mechanism by which PER2 is involved in controlling HMGB1 dependent neuroinflammation induced by sleep deprivation. Maintaining PER2 levels or blocking HMGB1 acetylation in the cortex might be prospective for preventing sleep deprivation-induced neuroinflammation and the related adverse reactions in the brain.
Keywords: PER2, HMGB1, Neuroinflammation, p300, Sleep deprivation
Subject terms: Biochemistry, Physiology
Introduction
Sleep deficit has become a wide spread problem in the current society1. Currently, the adverse health effects of circadian disruption caused by shift work, jet lag, or sleep deprivation have attracted widespread attention2. Plenty of research has revealed that insufficient sleep is correlated to the disorders in metabolic, immune, endocrine, respiratory and cardiovascular systems3–6. In addition, sleep deprivation may induce various brain dysfunction and cognitive impairment, including anxiety, depression, attention deficit, and working memory decline7,8. However, the mechanisms involved in sleep deficit-induced health risks are still far from being clarified. Neuroinflammation is widely induced in the central neural system (CNS) under multiple stress conditions, characterized by up-regulation of the pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF) α9. Neuroinflammation is considered as a common mechanism that contributes to various neurological disorders and cognitive impairments, mainly mediated by microglia and astrocytes10.
High-mobility group box 1 (HMGB1) is a non-histone chromosome-binding protein that can translocate from the nucleus into the cytoplasm and actively secrete into the extracellular space or passively release from the necrotic cells under certain stimuli11. Therefore, the intracellular function of HMGB1 is mainly determined by its subcellular distribution; while extracellular HMGB1 is regarded as a classic damage-associated molecular pattern (DAMP) that can induce immune and inflammatory responses by interacting with receptor for advanced glycosylation end products (RAGE), Toll-like receptor 4 (TLR4) and other cell surface receptors12–14. Posttranslational modifications (e.g. acetylation, ADP-ribosylation, phosphorylation, and methylation) have been shown to play a crucial role in the nuclear cytoplasmic shuttle and secretion/release of HMGB1 11,15. Among them, acetylation of lysine residues within the nuclear localization sequences (NLSs) is the most important posttranslational modification in regulating the nuclear export of HMGB1, whose responses are mediated by lysine acetyltransferase (such as p300, CBP) and deacetylase (such as histone deacetylase (HDAC) and sirtuin1 (SIRT1)) -dependent manners16,17. As one of the well-known mediators of neuroinflammation in CNS, HMGB1 is actively or passively released by damaged neurons, activated microglial and astrocytes or ruptured blood vessels18–21. Once entering cerebrospinal fluid, HMGB1 can bind with its receptors on glial cells and make them produce inflammatory factors (IL-1β, IL-6, etc.) and secrete more HMGB1, thereby exacerbating inflammatory cascades in CNS. Due to this reason, HMGB1 is considered as an amplifier of neuroinflammation22,23.
Sleep/wake cycle is regulated by two key factors throughout the 24 h a day: the circadian rhythm and the sleep/wake homeostasis24. Circadian rhythm is driven by the innate oscillations in organisms that are synchronized to the diurnal light/dark cycle on the earth. In mammals, the master internal pacemaker, which residents in the retinohypothalamic tract to hypothalamic suprachiasmatic nucleus (SCN), synchronizes peripheral clocks, which resident in the peripheral organs, and modulates circadian gene expression with light as the main Zeitgeber25. Both the master and peripheral clocks consist of an interlocking transcription-translation feedback loop (TTFL), which drives the circadian output of various biological functions, including sleep, metabolism, immune responses, etc26. Inside the cells, the basic helix–loop–helix (bHLH)–PER-ARNT-SIM (PAS) transcription factors CLOCK and BMAL1 activate the PER1, PER2, CRY1 and CRY2 genes, producing proteins that interact and repress their own transcription. Then ubiquitination-dependent degradation of PER and CRY proteins relieve their inhibitory effects on CLOCK and BMAL1, leading to the recovery of cellular and physiological functions driven by CLOCK and BMAL1 27,28.
In the current study, we investigated the influence of circadian clock genes on HMGB1 as a mechanism involved in neuroinflammation induced by sleep deprivation.
Materials and methods
Animals and treatment
Adult male Sprague-Dawley(SD)rats (6–8 weeks old, 180–200 g) were purchased from Charles River Laboratory Animal Technology Co. (Beijing, China), housed with food and water ad libitum and at consistent ambient temperature (21 ± 1℃) and humidity (50%±5%) with a 12 h light-dark cycle. The rats were randomly divided into control and sleep deprivation group (N = 6). To induce sleep deprivation model, rats were raised in the sleep deprivation device (SansBio Company, product model: SA109). The principle of this machine is to use a continuous rotating rod in a cage to keep the animals moving, preventing the experimental animals from falling asleep. It was also equipped with a computer system to precisely monitor the moving state of the machine. In our program, the whole sleep deprivation period was 72 h without interrupting, the dimensions of the cage is 400 × 400 × 170 mm and the rotating speed was 6.0 r/min. Rats studies were strictly complied with the ARRIVE guidelines and the care, use, and treatment of rats were in agreement with the National Research Council’s Guide for the Care and Use of Laboratory Animals. This study was approved by the Animal Ethics Committee of Beijing Institute of Basic Medical Sciences (No. IACUC-DWZX-2023-546).
Cell culture and transfection
The mouse microglial cells BV2 were purchased from ATCC. The mouse astrocyte C8D1A was kindly provided by Prof. Zhou Zhou (Zhejiang University, China). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10%FBS, 100 U/mL penicillin and streptomycin. LipofectAMINE 3000 (L3000075, Thermo Fisher Scientific) was used for FLAG-PER2 overexpression and LipofectAMINE™ RNAi MAX (13778150, Thermo Fisher Scientific) was used for p300 or PER2 siRNA transfection in the cells. All transfection reagents were used according to the manufacturer’s protocol. The plasmid expressing FLAG-PER2 was constructed by inserting the mouse PER2 cDNA into the pcDNA3.1-FLAG vector in our laboratory. Mouse p300 and PER2 siRNA were purchased from Riobo Biotechnology (Guangzhou, China). The siRNA sequences were as follows (5’ to 3’): p300: GCAAGCAGUCAUCUAUUUAdTdT (sense strand), UAAAUAGAUGACUGCUUGCdTdT (antisense strand);per2: GGACAUGAGACCAACGAAATTdTdT (sense strand), AAUUUCGUUGGUCUCAUGUCCdTdT (antisense strand). Cells were harvested 36 h after transfection for further analysis.
Western blot assay
Rats were anesthetized with 30 mg/kg pentobarbital sodium and the brain tissues were collected. Then the cortex and hippocampus were homogenized in protein lysis buffer containing a protease inhibitor cocktail. Proteins were subjected to western blot assay as described in our previous report29,30. Due to the diversity of the target proteins to be tested, the membrane was usually cropped into 2–4 parts based on the molecular weight of the target proteins and then the corresponding antibody hybridization was performed. The following primary antibodies were used in this study: anti-HMGB1(1:1000, ab18256, Abcam), anti-CLOCK (1:1000, 82829-1-RR, Proteintech), anti-BMAL1 (1:2500, 14268-1-AP, Proteintech), anti-CRY1 (1:1000, 13474-1-AP, Proteintech), anti-CRY2 (1:1000, 13977-1-AP, Proteintech), anti-PER2 (1:5000, GTX129688, GeneTex), anti-GAPDH (1:5000, 60004-1-IG, Proteintech), anti-β-actin (1:5000, 81115-1-RR, Proteintech), anti-LaminB1 (1:1000, 13435 S, Cell Signaling Technology), anti-SIRT1 (1:1000, 9475, Cell Signaling Technology), anti-p300 (1:200, sc-48343, Santa Cruz Biotechnology), anti-CBP (1:200, sc-365387, Santa Cruz Biotechnology), anti-FLAG (1:5000, F1804, Sigma-Aldrich), anti-acetylated-lysine (1:1000, 9441, Cell Signaling Technology), anti-β-tubulin (1:5000, R380628, ZEN-BIOSCIENCE), Goat Anti Mouse IgG-HRP (1:5000, P03S01, Gene-Protein Link), Goat Anti Rabbit IgG-HRP (1:5000, P03S02, Gene-Protein Link).
Immunoprecipitation
To test whether PER2 interacts with p300 in the microglial cells, BV2 cells were transfected with the plasmid expressing FLAG-PER2 or its vector. 36 h after transfection, cell lysates were collected, and immunoprecipitation assay was performed using anti-FLAG antibody. Then the immunoprecipitates were analyzed by using anti-p300, anti-PER2 and anti-FLAG antibodies. To determine the structural basis of PER2 involving in p300 interaction, several truncated PER2 mutants were constructed and transfected into BV2 cells. Then immunoprecipitation assay was performed using anti-FLAG antibody. Then the immunoprecipitates were analyzed by using anti-p300 and anti-FLAG antibodies. To test HMGB1 acetylation in the cortex of rats before and after sleep deprivation, the cortex lysates were immunoprecipitated with anti-HMGB1 antibody, and then the immunoprecipitates were analyzed by anti-acetylated-lysine antibody.
RNA isolation and RT-PCR assay
Total RNA was extracted from cortex by using TRIzol Reagent (T9424, Sigma-Aldrich) according to the manufacturer’s instructions, and cDNA was synthesized with the ThermoScriptTM RT-PCR system (Thermo Fisher Scientific). The primer sequences were used as follows (5’ to 3’): Gapdh: ACCCTTAAGAGGGATGCTGC (forward), GATGGGCTTCCCGTTGATGA (reverse);Hmgb1: ACTCGGAGAAACTTCAGACCG (forward), TGCATTGGGGTCCTTGAACT (reverse).
Cerebrospinal fluid HMGB1 levels assay
Cerebrospinal fluid (CSF) samples of control and sleep deprivation rats (N = 6) were collected and stored at − 80 °C until assayed. The HMGB1 levels in CSF were measured with the rat ELISA Kit (SEA399Ra, CLOUD-CLONE CORP) according to the manufacture’s protocol.
Nuclear cytoplasmic distribution of HMGB1 analysis
To determine the sub-cellular distribution of HMGB1 in the cortex of rats before and after sleep deprivation, the cortical nuclear and cytoplasmic extracts were prepared by using the Cytoplasmic/Nuclear Protein Extraction Kit (P0027, Beyotime Biotechnology) following the manufacturer’s instructions. Then the expression levels of HMGB1 in the different cellular compartments were determined by western blot assay.
Cellular NAD+ /NADH quantification assay
BV2 and C8D1A cells treated with EX527 (E7034, Sigma-Aldrich.) were collected and then subjected to cellular NAD+/NADH levels assay by using ELISA Kit (S0175, Beyotime Biotechnology) according to the manufacturer’s instructions.
Immunofluorescence assay
The rats with or without sleep deprivation were deeply anesthetized and then perfused with cold saline followed by 4% paraformaldehyde (PFA) (G1101, Service Biotechnology). The brains were dissected out, post-fixed in 4% PFA overnight at 4℃, and then dehydrated with 30% sucrose solution until the brains sank to the bottom. Optimal cutting temperature compound (OCT) (ZLI-9302, ZSGB-BIO) was used to embed the brains, which were sliced by using a Microtome Cyrostat. Sections (30 μm) were permeabilized and blocked with 3% BSA in 0.3%Triton X-100 (T8787, Sigma-Aldrich) containing PBS, then incubated with primary antibodies at 4℃ for 12 h. After extensive washing, they were incubated with fluorescent secondary antibodies at room temperature for 1.5 h, followed by nucleus staining with DAPI (C0065, Solar Biotechnology). The sections were visualized on Zeiss LSM980 confocal microscopes. The following primary antibodies were used in the Immunofluorescence assay: anti-HMGB1 (1:1000, ab18256, Abcam), anti-IBA1 (1:200, NB100-1028, Novus Biologicals), anti-GFAP (1:1000, 53-9892-82, Invitrogen), anti-NeuN (1:500, MA5-33103, Invitrogen), Goat anti-Rabbit IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 488 (1:500, A-11012, Invitrogen), Donkey anti-Goat IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 488 (1:1000, A-11055, Invitrogen), Goat anti-Chicken IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 488 (1:1000, A-11039, Invitrogen), Goat anti-Mouse IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 488 (1:1000, A-11001, Invitrogen).
Statistical analysis
Statistical analysis was conducted by using GraphPad Prism 8.0. Values are expressed as mean ± SD. Each independent experiment was performed with at least three biological replicates. When the two groups of samples conform to a normal distribution, the unpaired t-tests were utilized; when the two groups of samples do not conform to a normal distribution and the Wilcoxon rank-sum test were utilized. P Values were reported. Differences were considered statistically significant at P < 0.05.
Results
Sleep deprivation induced HMGB1 upregulation in the microglia of cortex and cerebrospinal fluids
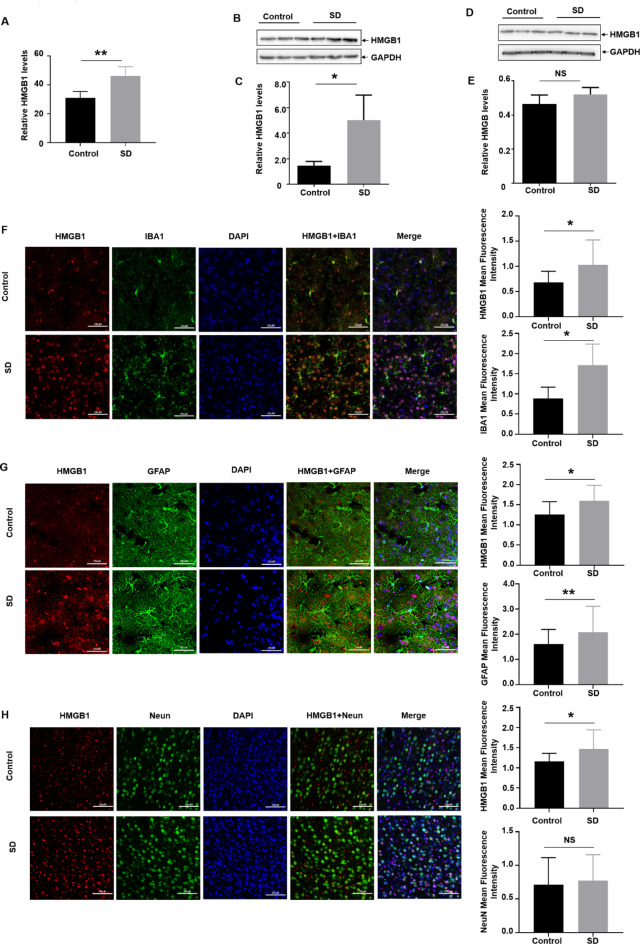
In the previous report, we revealed that three days sleep deprivation (SD) induced increased expression of the pro-inflammatory factors (IL-1β, IL-6 and TNFα) in both cerebrospinal fluids (CSF) and serum in the rats29. Due to important role of HMGB1 in mediating neuroinflammatory responses under various stress conditions31,32, we focused on investigating whether HMGB1 was also involved in neuroinflammation induced by sleep deprivation in this study. To this ends, 8-week-old male rats were subjected to SD for 72 h and then CSF and brain samples were collected to analyze the levels of HMGB1. Here we found that three days sleep deprivation induced elevation of HMGB1 levels in CSF (Fig. 1A), accompanying by more HMGB1 accumulation in the cortex (Fig. 1B and C), but not in the hippocampus (Fig. 1D and E). These data indicated that elevation of cortical HMGB1 expression might be a contributor in mediating neuroinflammation under the condition of insufficient sleep.
Fig. 1.
Sleep deprivation induced HMGB1 upregulation in the microglia of cortex and cerebrospinal fluids (CSF). 6-8-week-old male Sprague-Dawley rats were subjected to continuous sleep deprivation for 72 h, and then (A) HMGB1 levels in CSF before and after sleep deprivation were determined by ELISA. (N = 6) (Band D) HMGB1 levels in the cortex (B) and hippocampus (D) before and after sleep deprivation were detected by western blot assay. (N = 3) (C and E) Relative expression and quantification of the proteins in Fig. 1B (C) and 1D (E) were shown, respectively. (F–H) The expressions and quantification of HMGB1 in the cortical microglia (IBA-1-positive cells, F), astrocyte (GFAP-positive cells, G) and neuron (NeuN-positive cells, H) before and after sleep deprivation were detected by co-staining of HMGB1 with each cell marker as indicated in the immunofluorescence assay. Data are expressed as the mean ± SD. Scale bar: 25 μm; NS: no significance, * p < 0.05, **p < 0.01.
In our previous study, we have demonstrated that microglia and astrocyte can be activated in both cortex and hippocampus upon sleep deprivation, along with the increased expression in the pro-inflammatory factors (IL-1β, IL-6, TNF-α)29. Therefore, to elucidate the cellular origin of elevated HMGB1 expression in the cortex, we tested HMGB1 levels in cortical microglia and astrocyte before and after sleep deprivation by immunofluorescence staining assay. Because HMGB1 can be also secreted from the neuron33, we also detected neuronal HMGB1 levels in the cortex of the rats. Here, we found that the signal indicating HMGB1 expression was significantly enhanced after sleep deprivation, along with the morphological changes and increase in the numbers of IBA-1- and GFAP-positive cells (indicating activation of microglia and astrocytes, respectively). However, only co-staining of HMGB1 with IBA-1, but not with GFAP, was observed (Fig. 1F and G). Moreover, no signal indicated co-staining of HMGB1 with NeuN-positive cells (indicating neuron) (Fig. 1H). These data indicated that sleep deprivation-induced microglial activation produced more HMGB1, leading to elevation of this neuroinflammatory mediator in the rat cortex.
Sleep deprivation induced acetylation-dependent nuclear export of HMGB1 in the cortex
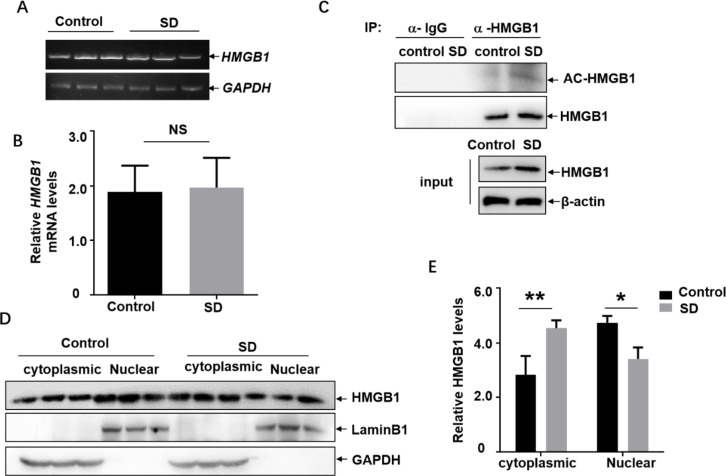
Next, we focused on investigating the mechanism involving in HMGB1 upregulation in the cortical microglia induced by sleep deprivation. Because we did not observe obvious difference on the HMGB1 mRNA levels in the cortex before and after SD (Fig. 2A and B), we believed that elevation of HMGB1 levels upon SD did not result from the transcriptional induction of HMGB1 mRNA. Then, we explored the possibility of post-translational modification (PTM) involving in mediating HMGB1 upregulation. Due to the important role of acetylation-dependent nuclear export and active secretion involving in HMGB1 upregulation under stress conditions34, cortex tissue lysate was subjected to immunoprecipitation with the anti-HMGB1 antibody and then blotted with anti-acetyl-lysine antibody to detect the possible changes on acetylation of HMGB1 with or without sleep deprivation. We found that the band signal indicating the acetylation level of HMGB1 significantly increased after sleep deprivation (Fig. 2C). Then, we compared the cytoplasmic/nuclear distribution of HMGB1 in the cortex tissue lysate before and after sleep deprivation. As shown in Fig. 2D and E, higher level of HMGB1 accumulated in the cytoplastic extracts of the cortical cells after sleep deprivation; while the nuclear HMGB1 levels decreased under the same conditions, indicating the translocation of HMGB1 from the nucleus to the cytoplasm occurred upon SD. Based on these data, we concluded that sleep deprivation induced acetylation-dependent nuclear export of HMGB1, which was a prerequisite for the HMGB1 upregulation and secretion.
Fig. 2.
Sleep deprivation induced acetylation-dependent nuclear export of HMGB1 in the cortex. (A) Rats were subjected to sleep deprivation for 72 h, and then the expression levels of cortical HMGB1 mRNA before and after sleep deprivation were detected with RT-PCR assay. (N = 3) (B)Relative expression and quantification of the mRNA in Fig. 2A were shown. (C) Rats were subjected to sleep deprivation for 72 h, and then cortex tissue lysate was subjected to immunoprecipitation with the anti-HMGB1 antibody followed by blotting with anti-acetyl-lysine antibody. (N = 3) (D) Rats were subjected to sleep deprivation for 72 h, and then the cytoplasmic/nuclear distribution of HMGB1 in the cortex tissue lysate before and after sleep deprivation were detected. (N = 3) (E) Relative expression and quantification of the proteins in Fig. 2D were shown. Data are expressed as the mean ± SD. NS: no significance, * p < 0.05, **p < 0.01.
p300 and SIRT1 regulated HMGB1 acetylation in the microglial cells
Next, we tried to figure out the upstream signaling pathway involving in enhanced acetylation of HMGB1 in the cortex upon sleep deprivation. According to the previous reports, the acetylation level of HMGB1 is modulated by both acetyltransferase (such as p300, CBP) and deacetylase (such as SIRT1)16,35. Therefore, the expression levels of these acetyltransferase and deacetylase were detected in the rat cortex before and after SD. We found that the levels of p300 and SIRT1 in cortex were significantly upregulated and downregulated upon SD, respectively (Fig. 3A, B and D); while CBP levels remained unchanged under the same condition (Fig. 3A and C). These data suggested that p300 and SIRT1 might be involved in modulating the acetylation of HMGB1 induced by SD. Next, p300 siRNA was transfected into the microglial cells BV2 to determine the possible contribution of p300 in regulating HMGB1 expression. We found that knockdown p300 levels resulted in a 5-fold down-regulation of HMGB1 expression in BV2 cells (Fig. 3E), indicating that microglia underwent p300-dependent HMGB1 acetylation. Then BV2 cells were exposed to EX527, the SIRT1 inhibitor, to determine the possible role of SIRT1 in regulating HMGB1 acetylation and expression. The effectiveness of EX527 in blocking the activity of SIRT1 was confirmed by a reduced intercellular NAD+/NADH ratio (Fig. 3F). Under these conditions, the expression of HMGB1 was upregulated 1.8-fold in BV2 cells (Fig. 3G). These results indicated that SIRT1 in the microglia were also functional in regulating HMGB1 expression. In the following study, we performed the same detection in the C8D1A astrocyte. However, HMGB1 levels showed slightly increase in C8D1A cells after knocking down p300 expression (1.3-fold of the control) (Fig. 3H); while slightly decrease under EX527 exposure (0.7-fold of the control) (Fig. 3I-J). These data further excluded the contribution of astrocyte to acetylation-dependent HMGB1 expression in the rat cortex upon SD.
Fig. 3.
p300 and SIRT1 regulated HMGB1 acetylation in the microglial cells. (A) Rats were subjected to sleep deprivation for 72 h, and then the expression levels of p300, CBP and SIRT1 in the cortex before and after sleep deprivation were detected. (N = 3) (B–D) Relative expression and quantification of the proteins in Fig. 3A were shown. (E and H) BV2 (E) or C8D1A (H) cells were transfected with p300 siRNA or its control siRNA, and then the expression levels of p300 and HMGB1 were detected at 36 h after transfection. (F and I) BV2 (F) or C8D1A (I) cells were treated with SIRT1 inhibitor EX527 or its solvent DMSO, and then the ratio of cellular NAD+/NADH was detected at 24 h later. (G and J) BV2 (G) or C8D1A (J) cells were treated as described in Fig. 3F and I, and then the expression levels of SIRT1 and HMGB1 were determined at 36 h later. Data are expressed as the mean ± SD. siRNA Transfection and inhibitor treatment in BV2 and C8D1A cells were repeated 3 times independently, the representative bands and their relative levels to the control bands are shown. NS: no significance, * p < 0.05, **p < 0.01.
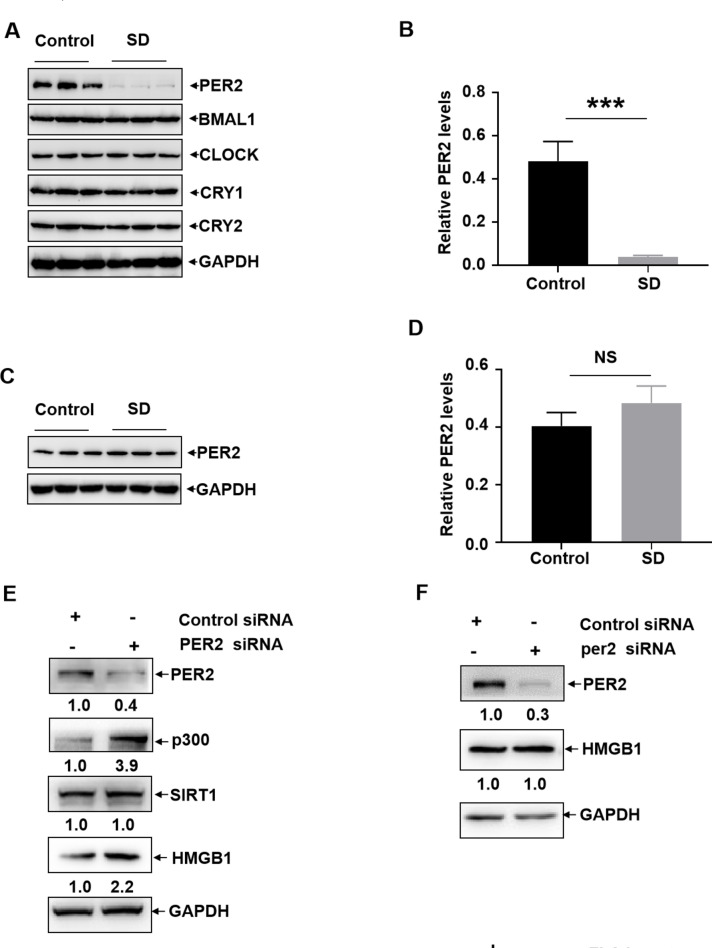
Impairment of the circadian clock protein PER2 expression resulted in p300-dependent HMGB1 upregulation in the microglia upon sleep deprivation
Due to the critical role of the circadian clock proteins in maintaining physiological homeostasis36, we next investigated whether sleep deprivation can induce the disruption of circadian clock proteins expression and contribute to HMGB1-related neuroinflammation. We found that the expression levels of PER2 reduced significantly in the cortex upon SD; while the cortical levels of other circadian clock proteins (CLCOK, BMAL1, CRY1, CRY2) remained unchanged under the same conditions (Fig. 4A and B). Consistent with the feature on the changes of HMGB1 expression, the levels of PER2 did not change in the hippocampus before and after sleep deprivation (Fig. 4C and D). These data indicated that PER2 downregulation, a specific response occurred in the cortex, might be involved in regulating HMGB1 expression upon SD. To address this possibility, PER2 siRNA was transfected into BV2 and C8D1A cells, respectively. We found that knockdown PER2 expression significantly upregulated HMGB1 as well as p300 levels in the BV2 cells, while the levels of SIRT1 did not show obvious changes under the same conditions (Fig. 4E). However, knockdown PER2 expression did not affect the levels of HMGB1 in C8D1A cells (Fig. 4F). These data indicated that p300-dependent HMGB1 acetylation was negatively controlled by PER2 in microglial cells.
Fig. 4.
Impairment of the circadian clock protein PER2 expression resulted in p300-dependent HMGB1 upregulation in the microglia upon sleep deprivation. (A) Rats were subjected to sleep deprivation for 72 h, and then the expression levels of the circadian clock proteins (CLCOK, BMAL1, PER2, CRY1, CRY2) in the cortex before and after sleep deprivation were detected. (N = 3) (B) Relative expression and quantification of the proteins in Fig. 4A were shown. (C) Rats were subjected to sleep deprivation for 72 h, and then the expression levels of PER2 in the hippocampus before and after sleep deprivation were detected. (N = 3) (D) Relative expression and quantification of PER2 in Fig. 4C were shown. (E and F) BV2 (E) or C8D1A (F) cells were transfected with PER2 siRNA or its control siRNA, and then the expression levels of PER2, p300, SIRT1 and HMGB1 were determined at 36 h later. Data are expressed as the mean ± SD. siRNA and plasmids transfection in BV2 cells were repeated 3 times independently, the representative bands and their relative levels to the control bands are shown. NS: no significance, * p < 0.05.
PER2 acted as a co-suppressor of p300 in microglial cells
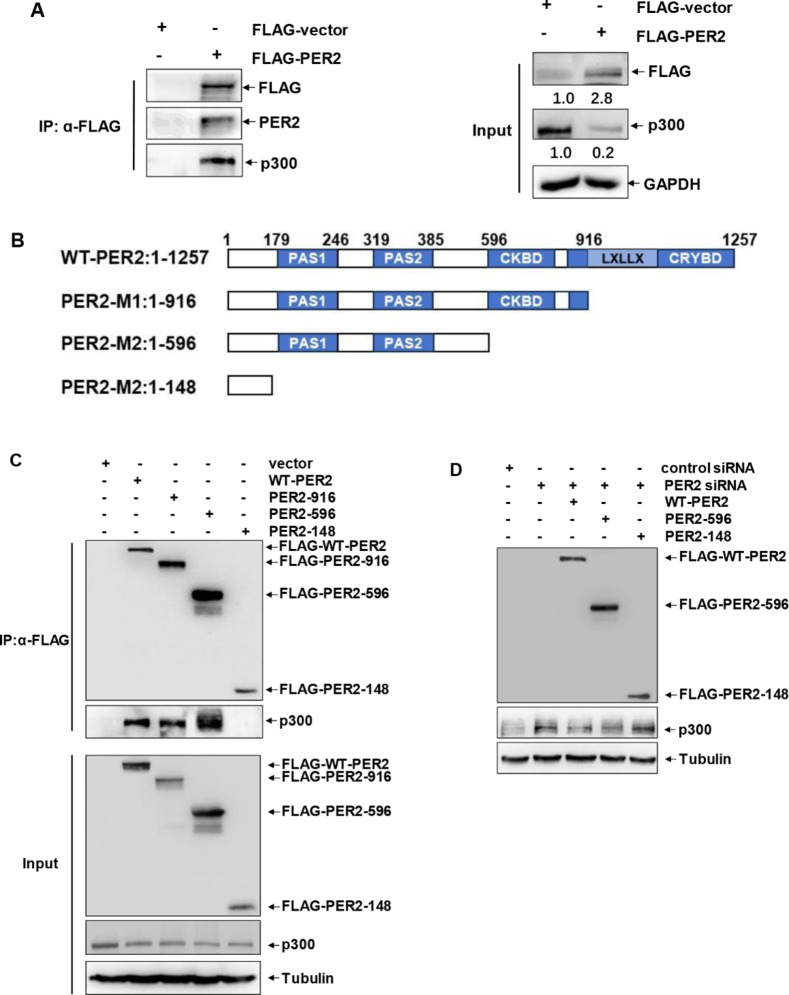
To further reveal the functional relationship between PER2 and p300 in mediating neuroinflammation, we next examined the possible interaction between these two molecules in microglia. As shown in Fig. 5A, ectopic expression of PER2 in BV2 cells significantly inhibited p300 expression, further confirming the negative role of PER2 in regulating the expression of p300. In addition, the interaction between PER2 and p300 was readily observed, indicating that PER2 acted as a co-suppressor of p300 in microglial cells. To reveal the structural basis of PER2 in inhibiting p300 expression, several truncated PER2 mutants were constructed, which successively deleted the CRY-binding domain (CRYBD), CK1δ-binding domain (CKBD), and PAS1/2 domains (Fig. 5B). When these mutants were transfected into BV2 cells, we found that N-terminal PER2 mutant (1-148aa) lacking CRYBD, CKBD and PAS1/2 domains lost its binding ability to p300, indicating that 149-1257aa in PER2 were involved in p300 interaction (Fig. 5C). Then we compared the ability of WT-PER2 and its mutants in regulating p300 expression. We observed that truncated PER2 mutant without p300 binding ability (1-148aa) could not exert the same function of suppressing p300 expression as its wild type counterpart (Fig. 5D), indicating that the interaction with p300 was critical for PER2 to exert its suppressing effect on p300 expression. Based on these results, we concluded that constitutive interaction between PER2 and p300 can be interrupted by PER2 downregulation upon sleep deprivation, leading to the increase in p300 expression, HMGB1 acetylation and secretion, and the elevation of HMGB1 levels in the cortex and CSF (Fig. 6).
Fig. 5.
PER2 acted as a co-suppressor of p300 in microglial cells. (A) BV2 cells were transfected with FLAG-PER2 expression plasmid or its control vector, and then the whole cell lysate were immunorepcipitated with anti-FLAG antibody followed by blotting with anti-p300 antibody to detect the potential interaction of PER2 and p300. (B) Design of the truncated PER2 mutants. (C) BV2 cells were transfected with WT-PER2 or the truncated PER2 mutants, and then the whole cell lysate were immunorepcipitated with anti-FLAG antibody followed by blotting with anti-p300 antibody to detect p300 binding domain within PER2. (D) BV2 cells were transfected with PER2 siRNA or its control siRNA, followed by reconstitution with WT-PER2 or the truncated PER2 mutants. Then the expression levels of p300 were detected at 36 h later.
Fig. 6.
Schematic presentation of working model in this study. PER2 constitutively interacted with p300 and inhibit p300 expressions in the cortical microglia under steady state. Sleep deprivation induced PER2 downregulation and interruption of PER2/p300 interaction. Then the increased expression of p300 triggered HMGB1 acetylation and secretion, leading to the elevation of HMGB1 in the cortex and CSF and the resultant neuroinflammation.
Discussion
Sleep deficit has been widely proved to be strongly associated with a series of physiological and cognitive decline in the brain, albeit the underlying mechanisms have not been well studied yet37. To perform the experiments in the current and our previous published studies, we used the commercial available sleep deprivation device to disrupt the sleep of mice/rats, and then both the mental and physical defects induced by sleep deprivation were observed29,30,38–40, which are consistent with the observations obtained in human studies41. So we believe that the animal model we used is effective and can at least extrapolate the experimental results to human in terms of physiological and cognitive impact. Of course, the sleep deprivation animal model we used also has certain limitations. For example, laboratory environments are highly controllable and lack the complex stimuli found in natural environments, such as social interactions and environmental changes. Another point is that mice have a shorter lifespan, and 72 h may have a more significant impact on their physiology. And 72 h in mice corresponds to several weeks in humans, and such a long period of sleep deprivation is obviously impossible to achieve in humans. So the significance of our results obtained by using this animal model is to predict the physiological and cognitive responses of humans under sleep deprivation.
According to our previous reports, sleep deprivation can induce markable increase in the production of pro-inflammatory factors such as IL-1β, IL-6, and TNFα, and further induce neuroinflammation in CNS29. As the resident macrophage in the brain, microglia can be activated by various stimuli and then differentiated into two different phenotypes, the pro-inflammatory M1 microglia or anti-inflammatory M2 microglia. Usually, M1 microglial cells express neurotoxic cytokines such as IL-1β, IL-6, and nitric oxide (NO); while M2 microglial cells express neuroprotective factors such as IL-4, IL-10, IL-13, and transforming growth factor-β (TGF-β), etc42. Similarly, astrocytes also exert both pro-inflammatory and neuroprotective effects in CNS through different phenotypes. The neurotoxic factors such as IL-1β, TNF-α, and NO can be secreted by pro-inflammatory A1 astrocytes; while the neuroprotective A2 astrocytes usually produce anti-inflammatory cytokines, such as IL-4, IL-13, and IL-1043.
In this study, we provided further evidence that insufficient sleep can significantly upregulate the level of HMGB1, one of the most important mediators of neuroinflammation, in cortical microglia and CSF. In fact, we also found a robust increase in serum HMGB1 levels upon sleep deprivation (data not shown). Although we currently cannot determine whether the accumulation of peripheral HMGB1 is caused by its leakage from CSF, it is certain that the elevated HMGB1 levels in the serum and CSF can serve as a biomarker indicating inflammatory response in the brain induced by sleep deprivation.
There are two main ways that are accepted for HMGB1 to be secreted to the extracellular space: passively release and actively secretion. In necrotic cells and nonlethal damaged cells, HMGB1 are passively released; while in immune responses, which mainly happens in immune cells, but also exist in other cell types such as neurons, HMGB1 are actively secreted33. Given that HMGB1 lacks of classical signal peptides, posttranslational modifications (e.g., acetylation, ADP-ribosylation, phosphorylation, and methylation) play crucial roles in the nuclear cytoplasmic shuttle and secretion/release of HMGB115. Among them, acetylation of lysine residues is most important in regulating the nuclear export of HMGB144. For example, in the lipopolysaccharide (LPS)-treated monocytes, acetylation of HMGB1 promote the dissociation of HMGB1 from the chromosome, leading to the cytoplasmic localization and secretion of HMGB145. In the current study, we proved that the increased expression of acetyltransferase p300 and the decreased expression of deacetylase SIRT1 synergistically enhanced the acetylation level of HMGB1 in the cortical microglial cells of rats after sleep deprivation, therefore triggered the nuclear export and secretion of this inflammatory factor. These data provided not only novel evidence for understanding the mechanism of neuroinflammation induced by sleep deficit, but also a prospective strategy for preventing sleep deprivation-induced neuroinflammation by applying inhibitor of HMGB1 or p300 or activator of SIRT1.
It is well-accepted that CSF HMGB1 is an amplifier of neuroinflammation, mainly due to its ability to act on various cells in the CNS and trigger inflammation related reactions. For example, HMGB1 can bind with its receptors on glial cells and make them produce inflammatory factors (IL-1β, IL-6, etc.) and secrete more HMGB1, thereby exacerbating inflammatory cascades in CNS42. In addition, elevated CSF HMGB1 can also act on neuron to induce their apoptosis22. With the neuronal death increased and the synaptosomes decreased in brain, more severe neuroinflammation and a significant damage to cognition such as Alzheimer’s disease (AD) or emotional disorders like depression may be induced or aggravated46. Moreover, HMGB1 releasing from neurons and glia during the acute phase of cerebral ischemia can upregulate hepcidin expression in astrocytes, leading to an iron surge and ferroptosis in neurons. Therefore, attenuation of HMGB1-dependent inflammatory response significantly reduces ferroptotic brain injury in the ischemic brain47. These observations together indicate that HMGB1 exerts a central role in forming a neuroinflammation-dependent vicious cycle between microglia, astrocyte, and neuron under various pathological conditions in the brain. Targeting HMGB1 might be critical to breaking the vicious cycle.
Although the link between circadian rhythm and neuroinflammation is well-accepted, the mechanistic understanding of their relationship is limited48. A previous study in the AD mouse model has revealed that the activation of neuroinflammation and downregulation of PER1, PER2, and Cry1 are involved in the pathogenesis of white matter lesions in AD49. Moreover, in the aged rats, aberrant PER1 and PER2 rhythms has been shown to be related to elevated cytokine expression in the microglia isolated from the hippocampus50. By contrast to the above studies that only disclose the phenotype of the relationship between circadian clock proteins and neuroinflammation, data in the current study have elucidated new signal transduction mechanism by which PER2 regulated neuroinflammation upon sleep deprivation. We have not only demonstrated the negative role of PER2 in regulating the expression of p300, the acetyltransfarase of HMGB1, in microglia, but also revealed the structural basis of PER2 in suppressing p300 expression. Therefore, we concluded that PER2 acted as a new co-suppressor of p300 in maintaining immune homeostasis in the brain, and dysregulation of PER2 led to the increased expression of p300 and HMGB1, and the resultant neuroinflammation. Based on these data, we have elucidated novel function of the circadian clock protein PER2 in controlling neuroinflammation by linking the acetylation-dependent signaling events, and provided new evidence for interpreting the adverse effects of sleep deprivation on the brain.
Although the structural and functional relationship of PER2 in inhibiting p300 expression has been well defined in the current study, the signaling pathway(s) leading to the suppression of p300 expression in the microglia are still unclear. Since we did not observe any changes in p300 mRNA levels in the cortex of rats after sleep deprivation or in BV2 cells with PER2 knockdown (data not shown), we speculated that downregulation of p300 expression might be caused by changes in the post-transcriptional or post-translational modification mechanisms controlled by PER2. Another interesting finding in this study was that PER2 did not show regulatory effect on the expression of the deacetylase SIRT1, which were also involved in HMGB1 acetylation and expression in the microglial cells. The downstream target selectivity of PER2 to deliver its regulatory role in protein acetylation is interesting to be clarified.
Another key point we have not addressed in this study is the potential mechanism leading to cortical PER2 downregulation after SD. In fact, the causes of changes in PER2 are pretty complex, potential mechanisms include alterations in neurotransmitters, dysregulation of endocrine hormones and the release of inflammatory factors. A previous study has shown that abnormally reducing melatonin could affect the normal rhythmic expression of circadian clock genes such as PER2, CRY2, BMAL1, CLOCK and PER1, while supplementing melatonin could regulate the expression of PER2 to maintain homeostasis51. Moreover, as a precursor to melatonin, 5-HT might be one of the factors contributing to changes of PER252. Another research revealed that lack of glucocorticoids might weaken the rhythmic expression of PER2, which could be restored by supplementing with glucocorticoids analog53. We also noticed that neuroinflammatory condition induced by LPS was demonstrated to be the cause of desynchronization of primary oscillators in SCN and subordinate brain oscillators in the hippocampus and cerebellum, accompanying with dysregulation of PER1 and PER2 immunoreactivities in these brain regions54. These results suggested that neuroinflammation might exert a feedback role in deregulating circadian clock proteins expression, thereby aggravate circadian disruption. Whether HMGB1 elevation induced by sleep deprivation also contributed to PER2 deregulation in the microglia is worthy to be defined.
Conclusions
In the current study, we demonstrated that PER2 constitutively interacted with p300 and inhibit p300 expressions in the cortical microglia under steady state. Sleep deprivation induced PER2 downregulation and interrupted PER2/p300 interaction, then the increased expression of p300 triggered HMGB1 acetylation and secretion, leading to the elevation of HMGB1 in the cortex and CSF (Fig. 6). Overall, these data reveal a new mechanism by which PER2 is involved in controlling HMGB1 dependent neuroinflammation by linking p300 upon sleep deprivation. Maintaining PER2 levels or blocking HMGB1 acetylation in the cortex might be prospective for preventing sleep deprivation-induced neuroinflammation and the related adverse reactions in the brain.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Min Zhang: Investigation, Methodology, Validation, Formal analysis, Visualization. Zhuoyao Ma: Writing-original draft, Validation, Visualization. Haoran Cui: Writing-original draft, Validation, Visualization. Yumeng Miao: Investigation, Validation, Visualization. Yu Yin: Investigation, Validation. Qing Wen: Investigation, Validation. Zhihui Liu: Investigation, Validation. Xin Huang: Investigation, Validation. Chen Xing: Resources. Kun Liu: Resources. Hui Peng: Resources. Lun Song: Conceptualization, Supervision, Project administration, Visualization, Writing-review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Key Research Project of China (XXXXX22 × 1031), Major Research Project of China (145XXXX05).
Data availability
The original data of western-blot assay and RT-PCR in this study were provided in the supplementary file.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the international guidelines for the care and use of laboratory animals. Approval was granted by the by the Animal Ethics Committee of Beijing Institute of Basic Medical Sciences (No. IACUC-DWZX-2023-546).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Min Zhang and Zhuoyao Ma contributed equally to this work.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-96931-6.
References
- 1.Perlis, M. L. et al. Insomnia Lancet400, 1047–1060 10.1016/s0140-6736(22)00879-0 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Fishbein, A. B., Knutson, K. L. & Zee, P. C. Circadian disruption and human health. J. Clin. Invest.13110.1172/jci148286 (2021). [DOI] [PMC free article] [PubMed]
- 3.Chaput, J. P. et al. The role of insufficient sleep and circadian misalignment in obesity. Nat. Rev. Endocrinol.19, 82–97. 10.1038/s41574-022-00747-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faraut, B., Boudjeltia, K. Z., Vanhamme, L. & Kerkhofs, M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep. Med. Rev.16, 137–149. 10.1016/j.smrv.2011.05.001 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Liew, S. C. & Aung, T. Sleep deprivation and its association with diseases- a review. Sleep. Med.77, 192–204. 10.1016/j.sleep.2020.07.048 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Tobaldini, E. et al. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci. Biobehav Rev.74, 321–329. 10.1016/j.neubiorev.2016.07.004 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Neculicioiu, V. S., Colosi, I. A., Costache, C., Sevastre-Berghian, A. & Clichici, S. Time to sleep?-A review of the impact of the COVID-19 pandemic on sleep and mental health. Int. J. Environ. Res. Public. Health. 19, 3497. 10.3390/ijerph19063497 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasula, E. Y. et al. Effects of sleep deprivation on component processes of working memory in younger and older adults. Sleep4110.1093/sleep/zsx213 (2018). [DOI] [PubMed]
- 9.DiSabato, D. J., Quan, N. & Godbout, J. P. Neuroinflammation: the devil is in the details. J. Neurochem. 139, 136–153. 10.1111/jnc.13607 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhusal, A., Afridi, R., Lee, W. H. & Suk, K. Bidirectional communication between microglia and astrocytes in neuroinflammation. Curr. Neuropharmacol.21, 2020–2029. 10.2174/1570159x21666221129121715 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lotze, M. T. & Tracey, K. J. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol.5, 331–342. 10.1038/nri1594 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Seong, S. Y. & Matzinger, P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol.4, 469–478. 10.1038/nri1372 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Paudel, Y. N. et al. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s disease (AD): from risk factors to therapeutic targeting. Cells9, 383. 10.3390/cells9020383 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang, H., Wang, H. & Andersson, U. Targeting inflammation driven by HMGB1. Front. Immunol.11, 484. 10.3389/fimmu.2020.00484 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, R., Kang, R. & Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med.54, 91–102. 10.1038/s12276-022-00736-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang, J. S. et al. Ligand-activated peroxisome proliferator-activated receptor-δ and -γ inhibit lipopolysaccharide-primed release of high mobility group box 1 through upregulation of SIRT1. Cell. Death Dis.5, e1432. 10.1038/cddis.2014.406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou, J. Y. & Crews, F. T. Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS One. 9, e87915. 10.1371/journal.pone.0087915 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou, S. S. et al. Brain microvascular endothelial Cell-Derived HMGB1 facilitates monocyte adhesion and transmigration to promote JEV neuroinvasion. Front. Cell. Infect. Microbiol.11, 701820. 10.3389/fcimb.2021.701820 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan, H. et al. Inhibiting HMGB1-RAGE axis prevents pro-inflammatory macrophages/microglia polarization and affords neuroprotection after spinal cord injury. J. Neuroinflammation. 17, 295. 10.1186/s12974-020-01973-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang, H., Andersson, U. & Brines, M. Neurons Are a Primary Driver of Inflammation via Release of HMGB1. Cells 10, 2791 (2021). 10.3390/cells10102791 [DOI] [PMC free article] [PubMed]
- 21.Wang, B. et al. Minocycline prevents the depressive-like behavior through inhibiting the release of HMGB1 from microglia and neurons. Brain Behav. Immun.88, 132–143. 10.1016/j.bbi.2020.06.019 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Wu, Z., Liang, L. & Huang, Q. Potential significance of high-mobility group protein box 1 in cerebrospinal fluid. Heliyon 9, e21926 (2023). 10.1016/j.heliyon.2023.e21926 [DOI] [PMC free article] [PubMed]
- 23.Wu, Z. & Li, M. High-Mobility group box 1 in spinal cord injury and its potential role in brain functional remodeling after spinal cord injury. Cell. Mol. Neurobiol.43, 1005–1017. 10.1007/s10571-022-01240-5 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuller, P. M., Gooley, J. J. & Saper, C. B. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J. Biol. Rhythms. 21, 482–493. 10.1177/0748730406294627 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Cheng, M. Y. et al. Prokineticin 2 transmits the behavioural circadian rhythm of the Suprachiasmatic nucleus. Nature417, 405–410. 10.1038/417405a (2002). [DOI] [PubMed] [Google Scholar]
- 26.Hurley, J. M., Loros, J. J. & Dunlap, J. C. Circadian oscillators: around the Transcription-Translation feedback loop and on to output. Trends Biochem. Sci.41, 834–846. 10.1016/j.tibs.2016.07.009 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamayo, A. G., Duong, H. A., Robles, M. S., Mann, M. & Weitz, C. J. Histone monoubiquitination by Clock-Bmal1 complex marks Per1 and Per2 genes for circadian feedback. Nat. Struct. Mol. Biol.22, 759–766. 10.1038/nsmb.3076 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirano, A. et al. FBXL21 regulates Oscillation of the circadian clock through ubiquitination and stabilization of Cryptochromes. Cell152, 1106–1118. 10.1016/j.cell.2013.01.054 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Xing, C. et al. Sleep disturbance induces depressive behaviors and neuroinflammation by altering the circadian oscillations of clock genes in rats. Neurosci. Res.171, 124–132. 10.1016/j.neures.2021.03.006 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Wang, W. et al. Circadian Oscillation expression of ornithine carbamoyltransferase and its significance in sleep disturbance. Biochem. Biophys. Res. Commun.559, 217–221. 10.1016/j.bbrc.2021.04.100 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Zhang, H., Ding, L., Shen, T. & Peng, D. HMGB1 involved in stress-induced depression and its neuroinflammatory priming role: a systematic review. Gen. Psychiatr. 32, e100084. 10.1136/gpsych-2019-100084 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stavely, R. et al. Oxidative Stress-Induced HMGB1 translocation in myenteric neurons contributes to neuropathy in colitis. Biomolecules12, 1831. 10.3390/biom12121831 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang, H. et al. HMGB1 released from nociceptors mediates inflammation. Proc. Natl. Acad. Sci. U S A. 118, e2102034118. 10.1073/pnas.2102034118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paudel, Y. N. et al. Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: updates on receptor signalling. Eur. J. Pharmacol.858, 172487. 10.1016/j.ejphar.2019.172487 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Yang, K. et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and Exosomal release in polymicrobial sepsis. Cell. Death Differ.29, 133–146. 10.1038/s41418-021-00841-9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer, N., Harvey, A. G., Lockley, S. W. & Dijk, D. J. Circadian rhythms and disorders of the timing of sleep. Lancet400, 1061–1078. 10.1016/s0140-6736(22)00877-7 (2022). [DOI] [PubMed] [Google Scholar]
- 37.Zielinski, M. R., Gibbons, A. J. & Neuroinflammation Sleep, and circadian rhythms. Front. Cell. Infect. Microbiol.12, 853096. 10.3389/fcimb.2022.853096 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xing, C. et al. Sleep disturbance induces increased cholesterol level by NR1D1 mediated CYP7A1 Inhibition. Front. Genet.11, 610496. 10.3389/fgene.2020.610496 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, W. et al. Sleep deprivation induced fat accumulation in the visceral white adipose tissue by suppressing SIRT1/FOXO1/ATGL pathway activation. J. Physiol. Biochem.80, 561–572. 10.1007/s13105-024-01024-z (2024). [DOI] [PubMed] [Google Scholar]
- 40.Xing, C. et al. Sleep deprivation reduced LPS-induced IgG2b production by up-regulating BMAL1 and CLOCK expression. Biochem. Biophys. Res. Commun.691, 149326. 10.1016/j.bbrc.2023.149326 (2024). [DOI] [PubMed] [Google Scholar]
- 41.Kecklund, G. & Axelsson, J. Health consequences of shift work and insufficient sleep. Bmj355, i5210. 10.1136/bmj.i5210 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Liang, F., Chen, S., Wang, Y. & Huang, Q. Glycometabolic reprogramming of microglia in neurodegenerative diseases: insights from neuroinflammation. Aging Dis.15, 1155–1175. 10.14336/ad.2023.0807 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwon, H. S. & Koh, S. H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 9, 42. 10.1186/s40035-020-00221-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu, B. et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc. Natl. Acad. Sci. U S A. 111, 3068–3073. 10.1073/pnas.1316925111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonaldi, T. et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. Embo J.22, 5551–5560. 10.1093/emboj/cdg516 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mangalmurti, A. & Lukens, J. R. How neurons die in Alzheimer’s disease: implications for neuroinflammation. Curr. Opin. Neurobiol.75, 102575. 10.1016/j.conb.2022.102575 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davaanyam, D. et al. HMGB1 induces Hepcidin upregulation in astrocytes and causes an acute iron surge and subsequent ferroptosis in the postischemic brain. Exp. Mol. Med.55, 2402–2416. 10.1038/s12276-023-01111-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muthukumarasamy, I., Buel, S. M., Hurley, J. M. & Dordick, J. S. NOX2 Inhibition enables retention of the circadian clock in BV2 microglia and primary macrophages. Front. Immunol.14, 1106515. 10.3389/fimmu.2023.1106515 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takase, H. et al. Transcriptomic profiling reveals neuroinflammation in the corpus callosum of a Transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis.97, 1421–1433. 10.3233/jad-231049 (2024). [DOI] [PubMed] [Google Scholar]
- 50.Fonken, L. K. et al. Diminished circadian rhythms in hippocampal microglia May contribute to age-related neuroinflammatory sensitization. Neurobiol. Aging. 47, 102–112. 10.1016/j.neurobiolaging.2016.07.019 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao, D. et al. Melatonin alleviates depression-like behaviors and cognitive dysfunction in mice by regulating the circadian rhythm of AQP4 polarization. Transl Psychiatry. 13, 310. 10.1038/s41398-023-02614-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi, X. et al. Hydroxytryptophan biosynthesis by a family of heme-dependent enzymes in bacteria. Nat. Chem. Biol.19, 1415–1422. 10.1038/s41589-023-01416-0 (2023). [DOI] [PubMed] [Google Scholar]
- 53.Liška, K., Sládek, M., Čečmanová, V. & Sumová, A. Glucocorticoids reset circadian clock in choroid plexus via period genes. J. Endocrinol.248, 155–166. 10.1530/joe-20-0526 (2021). [DOI] [PubMed] [Google Scholar]
- 54.Guissoni Campos, L. M. et al. Suprachiasmatic nucleus and subordinate brain oscillators: clock gene desynchronization by neuroinflammation. Neuroimmunomodulation24, 231–241. 10.1159/000484931 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data of western-blot assay and RT-PCR in this study were provided in the supplementary file.