Abstract
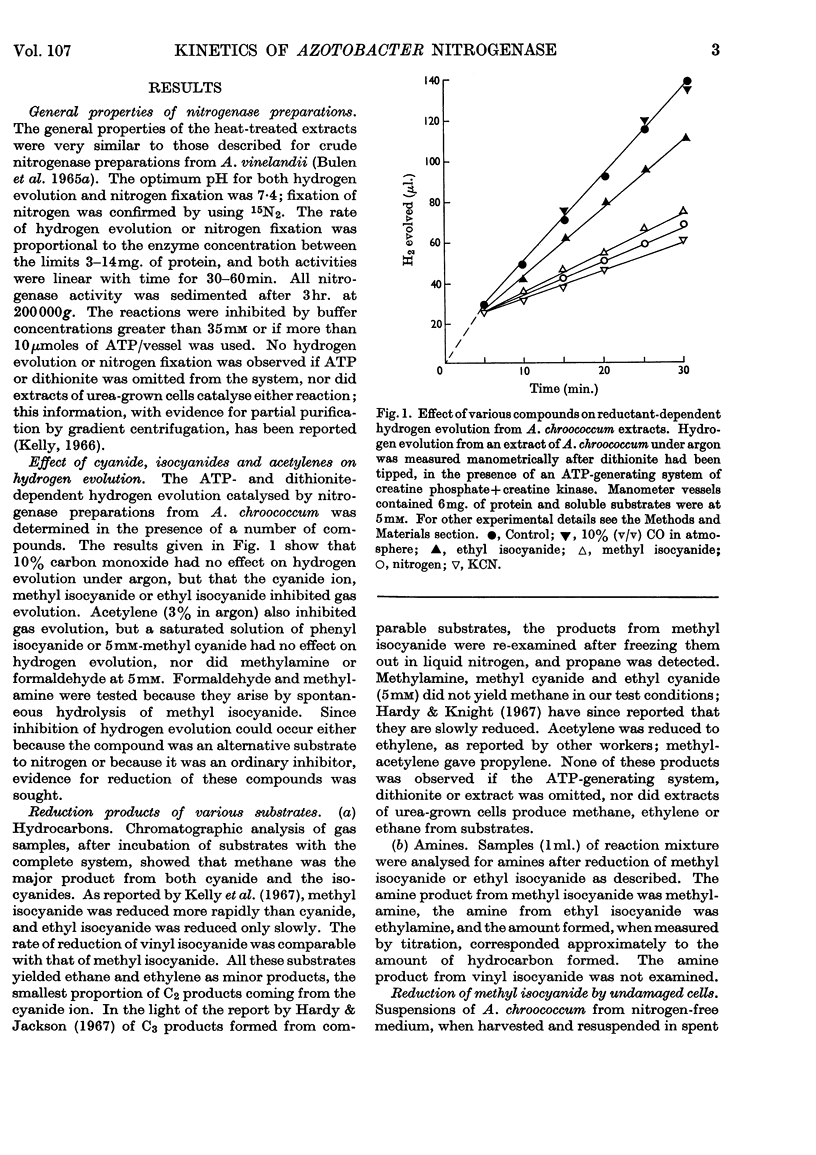
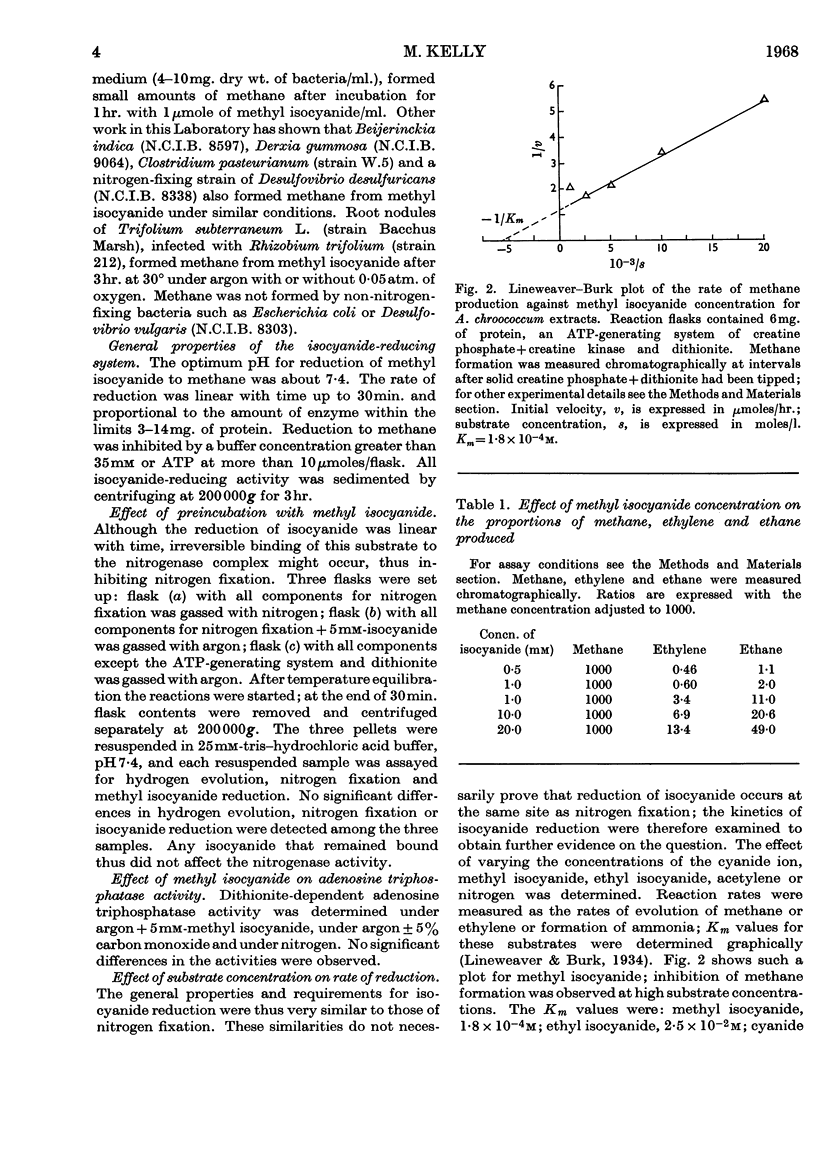
1. Nitrogen-fixing preparations from Azotobacter chroococcum reduced substrates with the following Km values: methyl isocyanide, 1·8×10−4m; ethyl isocyanide, 2·5×10−2m; cyanide ion, 1·4×10−3m; acetylene, 1·2×10−4m. 2. Nitrogen, carbon monoxide or hydrogen competitively inhibited isocyanide reduction with the following Ki values: hydrogen, 1·3×10−3m; carbon monoxide, 6·8×10−6m; nitrogen, 4·3×10−4m. 3. Living nitrogen-fixing bacteria, and isolated clover nodules, formed methane from methyl isocyanide. 4. These results are discussed in relation to other work and possible mechanisms of nitrogen fixation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULEN W. A., BURNS R. C., LECOMTE J. R. NITROGEN FIXATION: HYDROSULFITE AS ELECTRON DONOR WITH CELL-FREE PREPARATIONS OF AZOTOBACTER VINELANDII AND RHODOSPIRILLUM RUBRUM. Proc Natl Acad Sci U S A. 1965 Mar;53:532–539. doi: 10.1073/pnas.53.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARNAHAN J. E., MORTENSON L. E., MOWER H. F., CASTLE J. E. Nitrogen fixation in cell-free extracts of Clostridium pasteurianum. Biochim Biophys Acta. 1960 Nov 18;44:520–535. doi: 10.1016/0006-3002(60)91606-1. [DOI] [PubMed] [Google Scholar]
- Dilworth M. J. Acetylene reduction by nitrogen-fixing preparations from Clostridium pasteurianum. Biochim Biophys Acta. 1966 Oct 31;127(2):285–294. doi: 10.1016/0304-4165(66)90383-7. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Knight E., Jr Reduction of N2O by biological N2-fixing systems. Biochem Biophys Res Commun. 1966 May 25;23(4):409–414. doi: 10.1016/0006-291x(66)90742-x. [DOI] [PubMed] [Google Scholar]
- MORTENSON L. E. A simple method for measuring nitrogen fixation by cell-free enzyme preparations of Clostridium pasteurianum. Anal Biochem. 1961 Jun;2:216–220. doi: 10.1016/s0003-2697(61)80003-1. [DOI] [PubMed] [Google Scholar]
- NEWTON J. W., WILSON P. W., BURRIS R. H. Direct demonstration of ammonia as an intermediate in nitrogen fixation by Azotobacter. J Biol Chem. 1953 Sep;204(1):445–451. [PubMed] [Google Scholar]
- Proceedings of the biochemical society. Biochem J. 1967 Jan;102(1):1–11P. [PMC free article] [PubMed] [Google Scholar]
- Schöllhorn R., Burris R. H. Acetylene as a competitive inhibitor of N-2 fixation. Proc Natl Acad Sci U S A. 1967 Jul;58(1):213–216. doi: 10.1073/pnas.58.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöllhorn R., Burris R. H. Reduction of azide by the N2-fixing enzyme system. Proc Natl Acad Sci U S A. 1967 May;57(5):1317–1323. doi: 10.1073/pnas.57.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- WILSON T. G., ROBERTS E. R. Studies in the biological fixation of nitrogen. IV. Inhibition in Azotobacter vinelandii by nitrous oxide. Biochim Biophys Acta. 1954 Dec;15(4):568–577. doi: 10.1016/0006-3002(54)90015-3. [DOI] [PubMed] [Google Scholar]


