Abstract
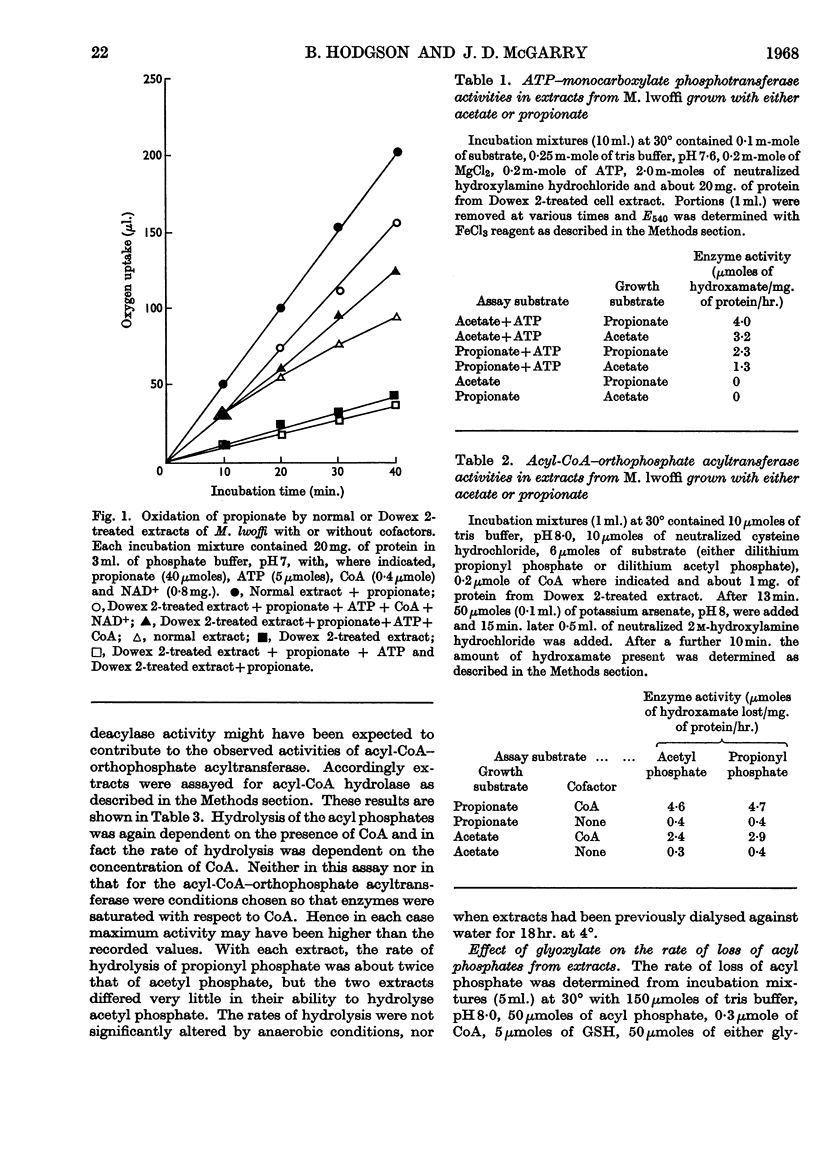
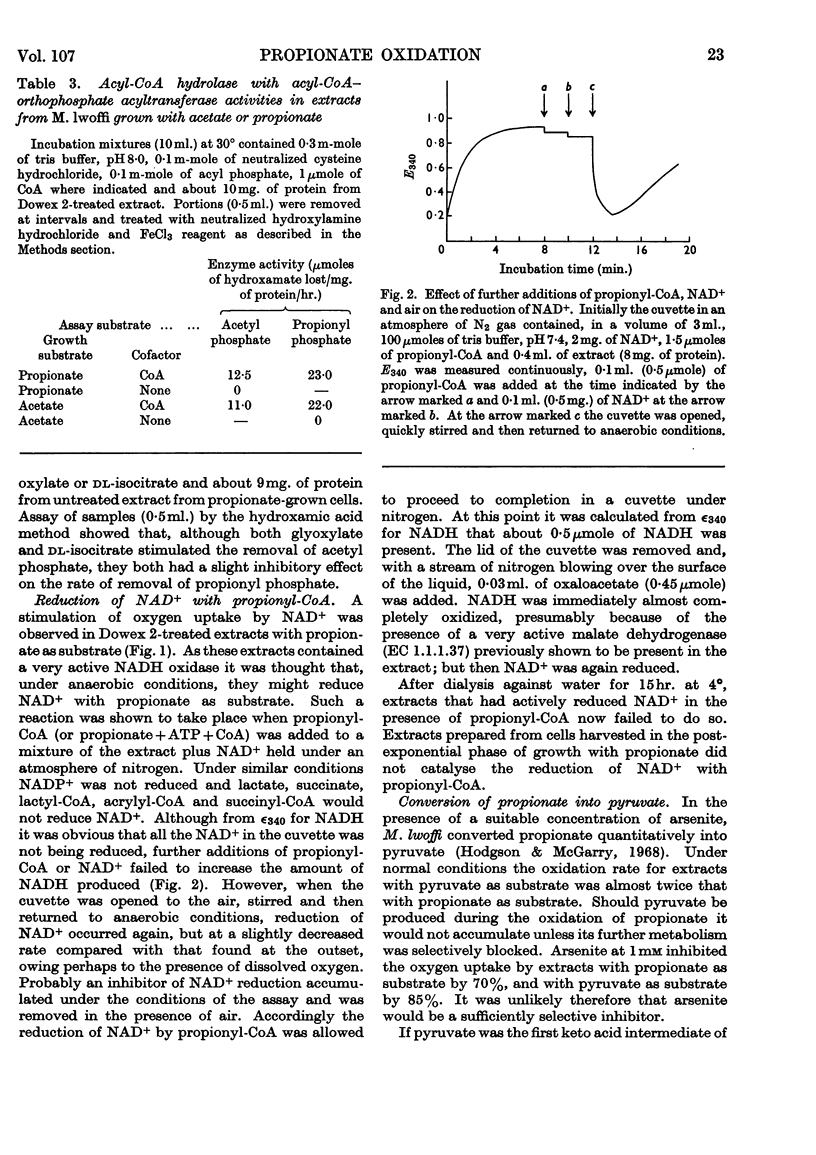
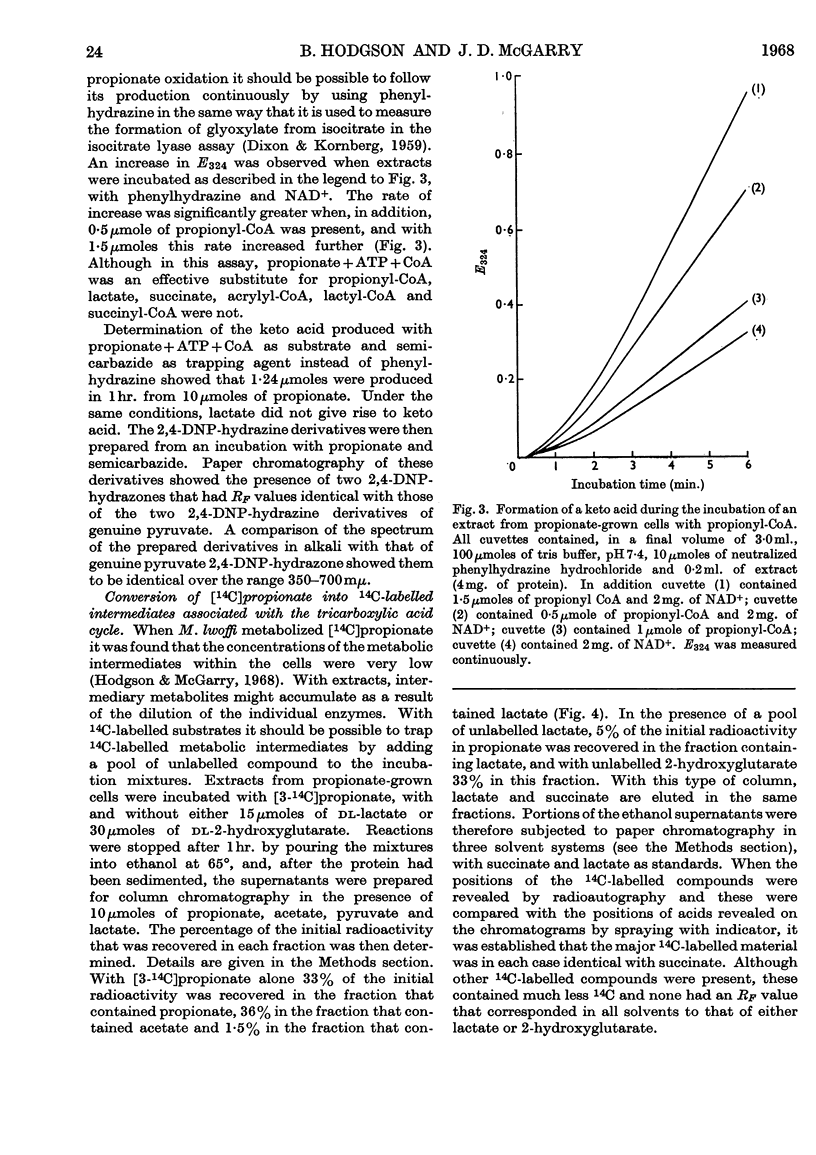
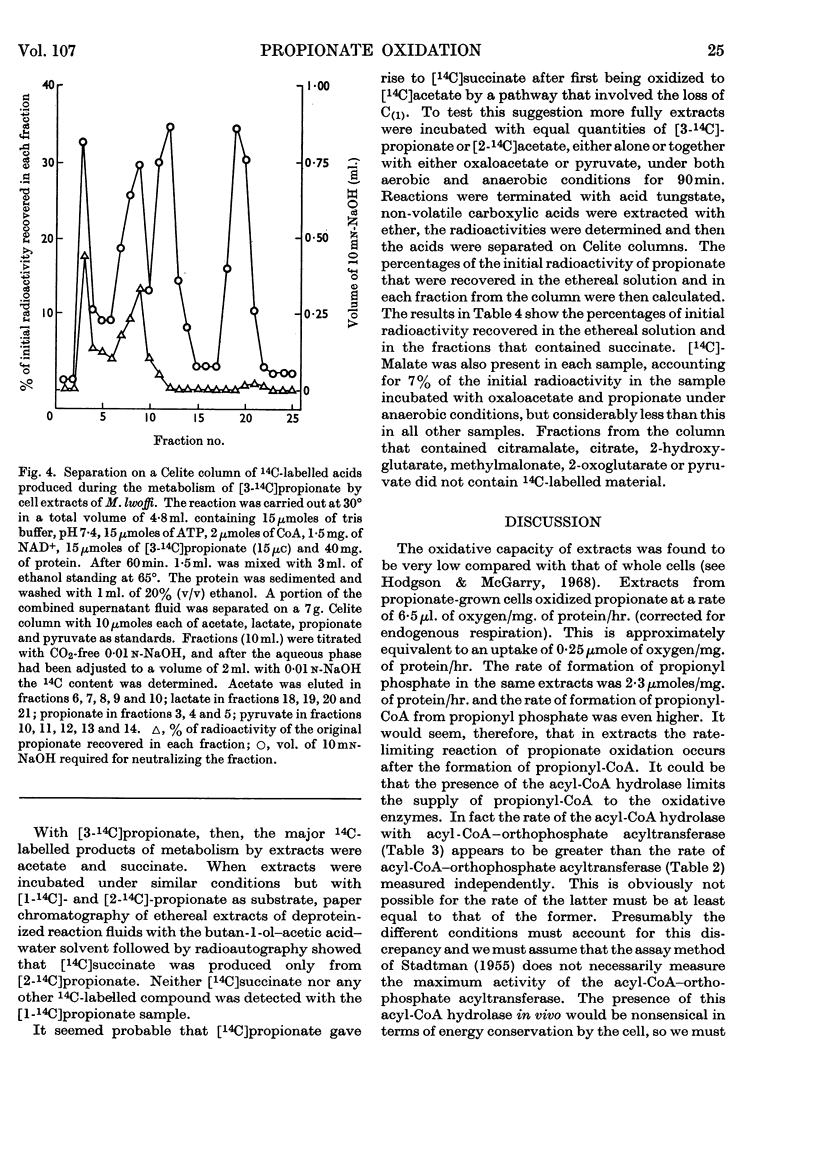
1. Extracts from Moraxella lwoffi oxidize propionate, but at a low rate when compared with whole cells. 2. This oxidative activity requires the formation of propionyl-CoA. 3. Enzymes catalysing the formation of propionyl phosphate and propionyl-CoA are present. The presence of a propionyl-CoA hydrolase is considered to be an artifact, but partly responsible for the low rates of oxidation. 4. Enzymes catalysing the reduction of NAD+ and the formation of pyruvate with propionyl-CoA as substrate are also present. 5. That the only pathway for the metabolism of propionate in extracts is a direct one to acetate via pyruvate was confirmed by the use of 14C-labelled materials. 6. A possible sequence of enzyme-catalysed reactions that will account for the experimental observations is described.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALDWIN R. L., WOOD W. A., EMERY R. S. LACTATE METABOLISM BY PEPTOSTREPTOCOCCUS ELSDENII: EVIDENCE FOR LACTYL COENZYME A DEHYDRASE. Biochim Biophys Acta. 1965 Feb 15;97:202–213. doi: 10.1016/0304-4165(65)90084-x. [DOI] [PubMed] [Google Scholar]
- CALLELY A. G., DAGLEY S., HODGSON B. Oxidation of fatty acids by cell-free extracts of a Vibrio. Biochem J. 1958 Jun;69(2):173–181. doi: 10.1042/bj0690173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANTRENNE H., LIPMANN F. Coenzyme A dependence and acetyl donor function of the pyruvate-formate exchange system. J Biol Chem. 1950 Dec;187(2):757–767. [PubMed] [Google Scholar]
- DAGLEY S., FEWSTER M. E., HAPPOLD F. C. The bacterial oxidation of aromatic compounds. J Gen Microbiol. 1953 Feb;8(1):1–7. doi: 10.1099/00221287-8-1-1. [DOI] [PubMed] [Google Scholar]
- Hodgson B., McGarry J. D. A direct pathway for the conversion of propionate into pyruvate in Moraxella lwoffi. Biochem J. 1968 Mar;107(1):7–18. doi: 10.1042/bj1070007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LADD J. N. The fermentation of lactic acid by a gram-negative coccus. Biochem J. 1959 Jan;71(1):16–22. doi: 10.1042/bj0710016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHLER H. R., HUENNEKENS F. M. The pathway of propionate oxidation. Biochim Biophys Acta. 1953 Aug;11(4):575–583. doi: 10.1016/0006-3002(53)90098-5. [DOI] [PubMed] [Google Scholar]
- OLSON J. A. The purification and properties of yeast isocitric lyase. J Biol Chem. 1959 Jan;234(1):5–10. [PubMed] [Google Scholar]
- STICKLAND L. H. The determination of small quantities of bacteria by means of the biuret reaction. J Gen Microbiol. 1951 Oct;5(4):698–703. doi: 10.1099/00221287-5-4-698. [DOI] [PubMed] [Google Scholar]
- SWIM H. E., KRAMPITZ L. O. Acetic acid oxidation by Escherichia coli; evidence for the occurrence of a tricarboxylic acid cycle. J Bacteriol. 1954 Apr;67(4):419–425. doi: 10.1128/jb.67.4.419-425.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAGELOS P. R. LIPID METABOLISM. Annu Rev Biochem. 1964;33:139–172. doi: 10.1146/annurev.bi.33.070164.001035. [DOI] [PubMed] [Google Scholar]


