Abstract
Background
Lysosomes are acidic organelles that play multiple roles in various cellular oxidative activities such as the oxidative burst during cytotoxic killing. It remains to be determined how lysosomal lumen oxidative activity and pH interact and are regulated. Here, I report the use of fluorescent probes to measure oxidative activity and pH of lysosomes in live macrophages upon treatment with the tumor promotor phorbol 12-myristate 13-acetate (PMA), and provide novel insight regarding the regulation of lysosomal oxidative activity and pH.
Results
The substrate used to measure oxidative activity was bovine serum albumin covalently coupled to dihydro-2', 4,5,6,7,7'-hexafluorofluorescein (OxyBURST Green H2HFF BSA). During pulse-chase procedures with live macrophages, this reduced dye was internalized via an endocytic pathway and accumulated in the lysosomes. Oxidation of this compound resulted in a dramatic increase of fluorescence intensity. By using low-light level fluorescence microscopy, I determined that phorbol ester treatment results in increased oxidative activity and pH elevation in different subsets of lysosomes. Furthermore, lysosomes with stronger oxidative activity tended to exclude the acidotropic lysosomal indicator, and thus exhibit higher alkalinity.
Conclusions
Results indicate that there is a regulatory mechanism between lysosomal oxidative activity and pH. Activation of lysosomal Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase by phorbol ester may result in increase of intralysosomal O2•- and H2O2, concurrent with pH elevation due to consumption of H+ and generation of OH-. Furthermore, the effect of phorbol ester on elevated oxidative activity and pH is heterogeneous among total lysosomal population. Higher oxidative activity and/or pH are only observed in subsets of lysosomes.
Background
The lysosome is a pivotal organelle responsible for a diverse spectrum of cellular functions including protein and lipid catabolism, vesicular transport and sorting [1]. Abnormal lysosomal physiology may result in cellular dysfunction, and eventually lead to a variety of diseases. For example, deficiencies of various enzymes responsible for lipid metabolism result in lipid accumulation in lysosomal storage diseases such as Niemann-Pick and Gaucher diseases [2]. Alternatively, modulation of lysosomal lumen pH may alter membrane trafficking and associated metabolic processes. It has been reported that elevated lysosomal pH is associated with the genetic lysosomal disorder, Mucolipidosis type IV disease where various sphingolipids and mucolipids accumulate in the lysosome [3]. Finally, lysosomes may play multiple roles in cellular oxidative metabolism [4]. The oxidative activity in lysosomes may regulate the modification of proteins and lipids, resulting in disruption of lysosomal membrane integrity [5].
The tumor promoter phorbol myristate acetate (PMA) has been shown to activate the NADPH (nicotinamide adenine dinucleotide phosphate) oxidase and initiate the oxidative burst response in various phagocytes, including neutrophils and the macrophage-like cell line J774A.1 [6,7]. During the oxidative burst, generation of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2) and superoxide anion (O2•-), is involved in degranulation, secretion of lysosomal enzymes and subsequent cytotoxic killing of invading pathogens. Although activation of the NADPH oxidase complex in phagolysosomal or lysosomal membranes has been reported, in situ generation of either H2O2 or O2•- in these organelles in live cells has not been demonstrated [6]. This is partly due to the lack of appropriate indicators that can monitor the formation of these ROS and the concurrent change of oxidative activity in these vacuoles. Furthermore, since the charged free radical O2•- should have difficulty leaking across hydrophobic membranes, formation of this anion radical inside the lysosome may have an immediate impact on pH in this organelle. The question addressed in the present study is: what is the distribution of oxidative activity and pH changes in the lysosome when cells are stimulated with PMA. A fluorogenic substrate, OxyBURST™ Green H2HFF Bovine Serum Albumin (OxyBURST Green H2HFF BSA), was used to detect lysosomal oxidative activity. OxyBURST Green H2HFF BSA consists of bovine serum albumin coupled to dihydro-2',4,5,6,7,7'-hexafluorofluorescein (H2HFF), a reduced dye with improved chemical stability. The non-fluorescent BSA conjugate is internalized into cells through the endocytic pathway, and accumulates in lysosomes where the extent of its oxidation depends on the oxidative activity in this organelle. The spatial distribution of lysosomal pH in cells after PMA treatment was investigated using both fluorescein-conjugated dextran and a selective lysosomal probe, LysoTracker™ Red. Finally, possible mechanisms of regulation between pH and oxidative activity in lysosomes are discussed.
Results and discussion
Effect of phorbol ester on the oxidative activities of lysosomes
Although the location of activated NADPH oxidase complex in phagolysosomes has been reported, the release of either H2O2 or O2•-in situ upon stimulation with PMA has not been directly shown in live cells [10,11]. In order to directly measure the oxidative activity in lysosomes, I use a reduced fluorescein derivative that is conjugated with BSA, OxyBURST Green H2HFF BSA, as shown in Figure 1. Spectrofluorimetric analysis indicates that this reduced compound is non-fluorescent, and becomes fluorescent upon HRP-catalyzed oxidation. Furthermore, upon incubation with macrophages, the reduced compound was internalized into cells through fluid phase endocytosis, and eventually accumulated in lysosomes. Therefore, the oxidation activity inside the lysosome determines the oxidation of the reduced dye. Upon oxidation, this compound emits strong fluorescence with emission maximum centered at λ545, which can be viewed and quantified using fluorescence microscopy.
Figure 1.
Structures and emission spectra of OxyBURST Green H2HFF BSA before (lower curve) and after (upper curve) oxidation in the presence of horseradish peroxidase (HRP) and hydrogen peroxide (H2O2).
I first labeled the total lysosomal population in cells with a well-known lysosomal marker, dextran conjugated with Cascade Blue fluorophore, according to a previously reported procedure [8] (see Methods section). Cells were then labeled with OxyBURST Green H2HFF BSA, followed by chase labeling without the reduced dye but in the presence or absence of 200 nM PMA. Co-localization experiments indicate that all of the OxyBURST Green H2HFF BSA is distributed in lysosomes but not endosomes after this labeling procedure (see below). The fluorescence intensity of the oxidized OxyBURST Green H2HFF BSA in total lysosomal populations was then quantified by a process using Cascade Blue-conjugated dextran as a mask for lysosomes [8]. As shown in Table 1, when cells were treated with PMA, fluorescence intensity of the OxyBURST fluorophore in lysosomes significantly increased (i.e. oxidative activity, 150% vs. 100%). This increase in fluorescence is not due to the internalization of oxidized dye since no oxidation of the OxyBURST Green H2HFF BSA was observed in the labeling medium during the loading procedure (data not shown). Furthermore, cells were incubated with the OxyBURST Green H2HFF at 37°C for 10 min so that the reduced dye is transported primarily into endosomes rather than lysosomes. Stimulation of endosome loaded cells with control medium or medium containing PMA resulted in no detectable changes in the fluorescent properties of the endosome population (data not shown). These results demonstrate that PMA initiates an elevation of oxidative activity selectively within lysosomes.
Table 1.
Effect of PMA on the oxidative activity and pH of total lysosomes
| Oxidative activitya (gray level/pixel area) | pHb | |
| Control | 82.1 ± 14.4 (100%) | 4.37 ± 0.05 |
| PMA | 123.9 ± 20.3 (150%) | 4.70 ± 0.21 |
a. Total lysosomal population was first labeled with Cascade Blue-conjugated dextran (10 K) and OxyBURST Green H2HFF BSA. The fluorescent intensities of oxidized OxyBURST in lysosomes of both control and PMA-treated cells were then measured as described. b. Lysosomal pH was measured using fluorescein-dextran in a dual-excitation ratiometric mode as described in Materials and method section.
In order to understand the in situ enhancement of lysosomal oxidative activity by PMA, the subcellular distribution of the oxidized OxyBURST Green H2HFF BSA in cells was investigated. As shown in Figure 2, oxidized OxyBURST Green H2HFF BSA distributed within the population of lysosomes where they co-localized with the fluorescent dextran. It was also noticed that strong fluorescence of oxidized OxyBURST Green H2HFF BSA only existed in a subset of lysosomes, as indicated by arrows in the inset.
Figure 2.
Oxidized OxyBURST Green H2HFF BSA distributes in a subset of lysosomes. The Cascade Blue-conjugated dextran was used as a lysosomal marker, and staining of OxyBURST Green H2HFF BSA in PMA-treated cells was performed as described in the Methods section. The area of a cell (indicated by the bracket) was enlarged for comparison, as shown in the inset of each panel. As indicated by the arrows, oxidized dye preferentially accumulates in a subset of lysosomes. Bar, 10 μm.
Effect of phorbol ester on lysosomal pH
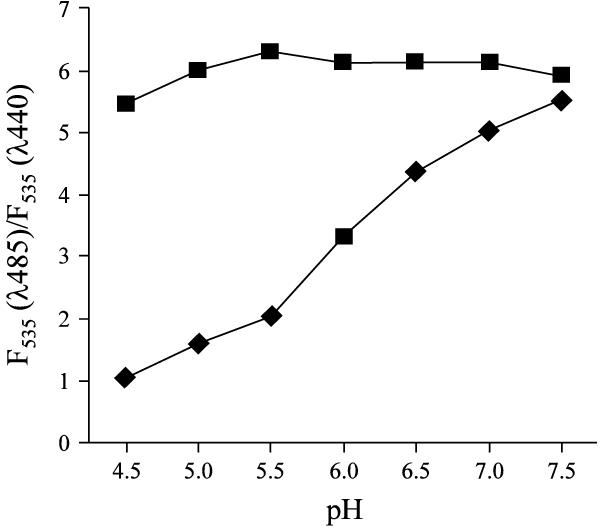
I next sought to investigate the effect of PMA treatment on lysosomal pH. Averaged lysosomal pH was measured in control cells or cells treated with PMA, using fluorescein-conjugated dextran in dual-excitation ratiometric mode [8]. The validation of lysosomal pH measurements was performed by the calibration curve using ionophore-clamped cells as shown in Figure 3. The ratio of emission fluorescence under excitation at 485 nm versus 440 nm (i.e. F535(λ485)/F535 (λ440)) correlated linearly with calibrated lysosomal pHs from 4.0 to 6.5, with a high r2 value of 0.9689. As shown in Table 1, the average pH in total lysosomal population significantly increased (0.1–0.6 pH units) upon PMA stimulation. Most interestingly, a heterogeneous distribution of lysosomes with elevated pH was observed by ratiometric images as shown in Figure 4. Here, lysosomal pH in untreated cells is very homogeneous, and estimated to be 4.3–4.5. Upon PMA treatment, pH was significantly elevated to as high as 6.5–7.5 in a subset of lysosomes (indicated by the arrow).
Figure 3.
In vivo pH calibration curve of fluorescein-dextran (F535(λ485)/F535(λ440) ratio against pH). Lysosomes of live J774A.1 cells were labeled with fluorescein-dextran and then incubated with MES calibration buffer solutions (pH from 4.0 to 7.0) containing nigericin and monensin. Fluorescence images of emission wavelength at 515–565 nm were acquired under the excitation at 485 and 440 nm (i.e. F535(λ485) and F535(λ440), respectively). The ratio of F535(λ485) over F535(λ440) in the lysosomes was then calculated as described in the Methods section.
Figure 4.
PMA treatment increases pH to 6.5–7.5 in a subset of lysosomes. Lysosomal pH in untreated cells is below 4.5 as shown on the left image. In PMA-treated cells, pH was significantly elevated to as high as 6.5–7.5 in only a subset of lysosomes as indicated by the arrow, while the pH of other lysosomes were essentially unaffected. Nu, nucleus. Bar, 10 μm.
The effect of PMA-induced oxidation on lysosomal pH as measured by the fluorescein-dextran is worthwhile further discussion (see Table 1 and Figure 4). It is critical to test the possibility that the increase of oxidative activity may change the pH-dependence of fluorescein-dextran. In other words, lysosomes with higher oxidative activities may induce abnormal change of the pH-dependence of fluorescein-dextran that is within. If this is true, then the resulting F535(λ485)/F535 (λ440) ratio of the fluorescence is no longer pH-dependent, and can not be used as an indication of pH increase in lysosomes. To test this possibility, effect of HRP-catalyzed oxidation on pH-dependence of fluorescein-dextran was investigated in vitro. As shown in Figure 5, increase in oxidation showed no effect on the pH-dependence of fluorescein-dextran. Conversely, changes in lysosomal pH do not affect the fluorescence of oxidized OxyBURST Green H2HFF BSA as will be shown later.
Figure 5.
Effect of HRP-catalyzed oxidation on the pH-dependency of fluorescein-dextran fluorescence. Fluorescein-dextran (final concentration at 30 μg/ml) was prepared in MES calibration buffers with pHs ranging from 3.0 to 7.3. These solutions were then incubated at 37°C for 30 min, in the absence (●) or presence (○) of H2O2 and HRP (final concentrations are 1 μM and 4 μg/mL, respectively). The fluorescence emission ratio of F535(λ485)/F535 (λ440) was then measured.
Regulation of lysosomal pH and oxidative activities
The observation that there are elevations of both oxidative and pH activities in only a subset of lysosomes (Figures 2 and 4) suggests that pH and oxidative activity may be coupled in this organelle. In order to demonstrate this relationship in live cells, the oxidative activities and pH in lysosomes were simultaneously monitored by OxyBURST Green H2HFF BSA and LysoTracker Red DND-99 which is able to qualitatively indicate acidic lysosomes.
Briefly, cell monolayers were incubated with 200 μg/mL OxyBURST Green H2HFF BSA at 37°C for 2 hours, washed, and then incubated in fresh medium containing 200 nM PMA at 37°C for 0.5 hour. Cell monolayers were washed and then incubated with 25 nM LysoTracker Red DND-99, at 37°C for 10 min, to label acidic lysosomes. Consistent with previous results, PMA treatment elicits high oxidative activity and thus increased OxyBURST fluorescent intensity in a subset of lysosomes (Figure 6). Interestingly, lysosomes with strong OxyBURST fluorescence contain only very weak fluorescence of LysoTracker Red (see arrows). On the other hand, acidic lysosomes strongly stained by LysoTracker Red exhibited very weak OxyBURST fluorescence (see arrowhead). The mutual regulation of oxidative activity and alkalinity in lysosomes can be better demonstrated by a ratio image as shown in the lower panel.
Figure 6.

Oxidative activity is high in lysosomes that are weakly stained by the acidotropic dye LysoTracker Red DND-99. Cell monolayers were incubated with 200 μg/mL OxyBURST Green H2HFF BSA at 37°C for 2 hours, washed, and then incubated in the fresh medium containing 200 nM PMA at 37°C for 0.5 hour. Cell monolayers were washed and then incubated with 25 nM LysoTracker Red DND-99, at 37°C for 10 min, to label acidic lysosomes. As indicated in the two upper panels, high OxyBURST fluorescent intensity and thus high oxidative activity are observed in a subset of lysosomes which contain weak fluorescence with the LysoTracker Red and hence low acidity (indicated by arrows). On the other hand, lysosomes strongly stained by the LysoTracker Red only exhibit lower oxidative activities (see arrowheads). The lower panel is the resulting ratio image of the OxyBURST over LysoTracker Red fluorescent intensities, which represents the spatial distribution of an arbitrary oxidative activity/alkalinity ratio in the same cell. Bar, 10 μm.
LysoTracker Red DND-99 is a chloroquine analogue that had been reported to selectively accumulate in acidic lysosomes [9]. In the present study, when lysosomal pH was elevated to >6.5 and then cell was stained with the dye, the fluorescence in lysosomes greatly decreased or diminished. This decrease in fluorescence is due to a decrease in accumulation of the dye in lysosomes since the change in pH itself has no effect on the fluorescence of the dye (data not shown). Nevertheless, the fluorescence decrease of this dye in a subset of lysosomes after PMA stimulation could also result from reactions of the LysoTracker Red with higher oxidative activity, while the pH in this subset of lysosomes remains unchanged. This possibility was tested in vitro by incubating LysoTracker Red with H2O2 or KO2 (as an O2- donor) in buffer solutions with acidic (4.5) or alkaline (7.5) pHs. As shown in Figure 7, the fluorescence of the LysoTracker Red is not affected by either of these two oxygen species. This data strongly supports the contention that decreased fluorescence of the LysoTracker Red in a subset of lysosomes does indicate elevation of pH.
Figure 7.
The effect of H2O2 and O2- on the fluorescence of LysoTracker Red DND-99. 2 μM of LysoTracker Red DND-99 was prepared in MES buffer solutions at either pH 4.5 (panel A) or pH 7.5 (panel B). H2O2 (◆) or KO2 (■) were then added to various final concentrations and the resulting reaction solutions were incubated at room temperature for 10 min. Fluorescence of the LysoTracker Red was then determined.
I then characterized the heterogeneous fluorescence distribution of oxidized OxyBURST Green H2HFF BSA. Fluorescence of fluorescein and its analogs such as carboxyfluorescein has been shown to be pH-dependent [10,11]. Elevation of pH increases the ratio of fluorescent emission at λ535 as excited by λ485 over λ440. The heterogeneous fluorescence of the oxidized dye in subsets of lysosomes where it exhibits a significant difference in pH (4.5 to 7.5) may simply reflect the pH-dependency of the oxidized dye, and not represent different levels of oxidative activity among lysosomes. The pH-dependency of fluorescence of both oxidized OxyBURST Green H2HFF BSA and 5-(and -6)-carboxyfluorescein were thus examined by the dual excitation ratiometric measurement in vitro, as shown in Figure 8. While 5-(and -6)-carboxyfluorescein exhibits strong pH-dependency, the experiment clearly demonstrates that fluorescence of the oxidized OxyBURST Green H2HFF BSA is not affected by variation of the pH within a range between 4.5 to 7.5.
Figure 8.
Effect of pH on the fluorescence of oxidized OxyBURST Green H2HFF BSA. Oxidized OxyBURST Green H2HFF BSA (■) and 5-(and -6)-carboxyfluorescein (◆) were prepared in MES buffer solutions at various pHs, to a final concentration of 78 μg/ml and 4.2 μM respectively. The ratio of emission fluorescence at λ535 upon excitations with λ485 over λ440 (i.e. F535(λ485)/F535 (λ440)) was then determined.
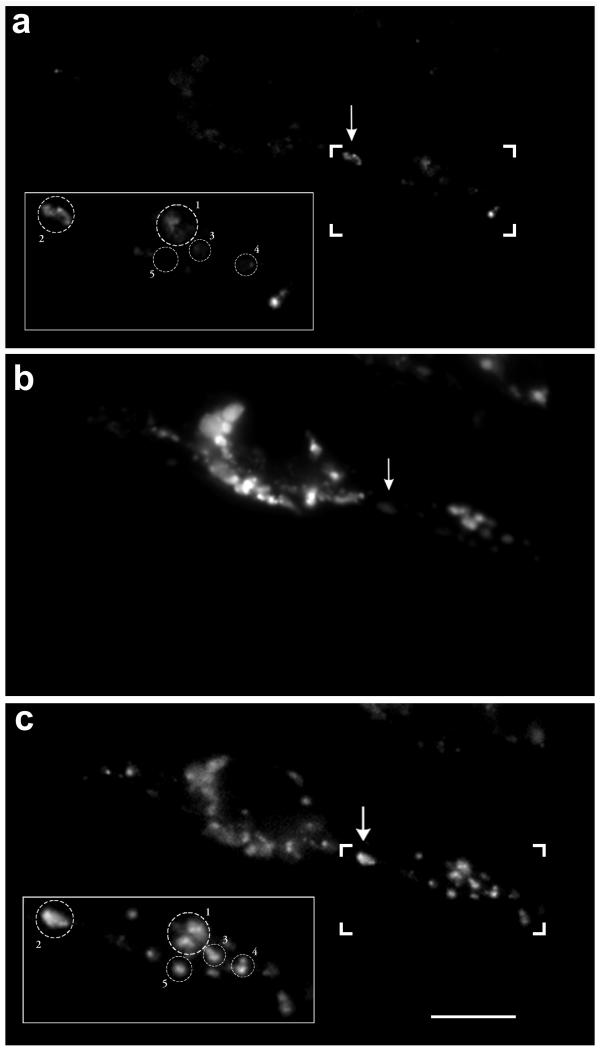
It is also possible that the heterogeneous distribution of fluorescence intensity of oxidized OxyBURST Green H2HFF BSA is due to preferential accumulation of the reduced dye in a subset of lysosomes rather than being determined by a variation of oxidative activity in these lysosomes. In other words, lysosomes that accumulate more reduced dye will produce stronger fluorescence after PMA stimulation. To address this possibility, I performed the following experiment. Cell monolayers were grown on circular glass coverslips and labeled with OxyBURST Green H2HFF BSA as previously described. The cells were then mounted onto a temperature-controlled stage with a perfusion chamber for microscopy. The temperature was controlled at 37°C throughout the experiment. Cells were then treated with 200 nM PMA for 30 min, followed by 25 nM LysoTracker Red DND-99 for 5 min to label lysosomes. Cells were next washed with PBS and the fluorescence images of both oxidized OxyBURST Green H2HFF BSA and LysoTracker Red were recorded. As shown in Figure 9a, the fluorescence intensity is higher in a subset of lysosomes (see arrow) that accumulate very little LysoTracker Red (Figure 9b). Conceivably, if there was more reduced dye in this lysosome area, complete oxidation of the reduced dye inside lysosomes should release more OxyBURST fluorescence. This was tested by adding H2O2 to the cell (final concentration of 0.05% in PBS). The cells were then incubated for an additional 15 min to allow oxidation of all the lysosomal OxyBURST Green H2HFF BSA. The fluorescent image of oxidized OxyBURST Green H2HFF BSA in the same cell was then recorded as shown in Figure 9c. The addition of H2O2 resulted in a 4.5-fold average intensity increase (349.5 vs. 73.5) among the total lysosomal population. Fluorescence changes in five selected lysosomal areas before (insets, Fig. 9a) and after (insets, Fig. 9c) H2O2 addition are further quantified as shown in Figure 10. The figure shows that the maximal fluorescence released by H2O2treatment among these five lysosomal areas is very similar (421.21–455.81 gray level/pixel area). Importantly, lysosomal area 2, which exhibited stronger fluorescence before H2O2 treatment (175.12 gray level/pixel area), does not release more fluorescence after complete oxidation by the peroxide. This clearly eliminates the possibility that there is more OxyBURST Green H2HFF BSA accumulating in lysosome area 2. The experiment strongly supports the notion that the oxidative activity within the lysosomal population is heterogeneous.
Figure 9.
Heterogeneous distribution of oxidative activity in lysosomes is not due to heterogeneous distribution of the OxyBURST Green H2HFF BSA Cell monolayers were grown on circular glass coverslips and labeled with OxyBURST Green H2HFF BSA as described. The cells were then mounted onto a temperature control stage with a perfusion chamber for microscopy. The temperature was controlled at 37°C throughout the experiment. Cells were then treated with 200 nM PMA for 30 min, followed by 25 nM LysoTracker Red DND-99 for 5 min. Cells were then washed with PBS and the fluorescence images of both oxidized OxyBURST Green H2HFF BSA and LysoTracker Red were acquired. As shown in the panel A, oxidation activity is high in a subset of lysosomes (a, see arrow), which accumulate little LysoTracker Red (b). To oxidize all the OxyBURST Green H2HFF BSA in the same cells, H2O2 was added to the chamber to a final concentration of 0.05% in PBS. The cells were then incubated for an additional 15 min to allow oxidation of all the lysosomal OxyBURST Green H2HFF BSA. Afterwards, the fluorescent image of oxidized OxyBURST Green H2HFF BSA from the same cell was then recorded as shown in (c). Bar, 10 μm.
Figure 10.
The change of fluorescence intensity in five selected lysosomal areas indicated in insets of Figure 7a (pre-H2O2) and Figure 7c post-H2O2).
One concern regarding to the use of OxyBURST Green H2HFF BSA in the present study is that the accumulation of OxyBURST Green H2HFF BSA itself may drive the increase in pH. If this is the case, then lysosomes containing OxyBURST Green H2HFF BSA should have more alkaline pH. However, this possibility is not consistent with one observation that was presented in Figure 9 where I showed that distribution of OxyBURST Green H2HFF BSA was very homogenous among lysosomes, nevertheless not every lysosomes exhibited elevated lumen pH.
Although direct measurements of lysosomal oxidative activity in live cells were not available before the present study, several lines of evidence did point to a regulatory mechanism in phagolysosomes or lysosomes in phagocytes during phagocytosis and oxidative burst. First, it has been shown that activation of NADPH oxidase during a phagocytosis event results in alkalization of phagolysosomal pH in the human neutrophil [10]. The phagolysosomal pH is influenced by NADPH oxidase activity and to a lesser extent by a Na+/H+ antiporter. Secondly, disruption of normal lysosomal pH by lysosomotropic weak bases inhibits the respiratory burst in neutrophils [12]. A normal transmembrane pH gradient in lysosomes is suggested to play an important role in assembling the NADPH oxidase complex during cell activation. These observations and my present results can be integrated and summarized in a model proposed in Figure 11. PMA treatment induces the assembly and activation of NADPH oxidase in the lysosomal membrane. Activation of the oxidase generates intralysosomal O2•- that is membrane impermeable, with a short life span and is converted to H2O2 instantly by the consumption of protons (see (1)). H2O2 then oxidizes the OxyBURST Green H2HFF BSA that accumulates in the lysosome in the presence of peroxidases. This enzyme-catalyzed oxidation generates hydroxyl ion (OH-) and radical (OH) (see (2)). Intralysosomal pH elevation can be explained by a net effect of H+ consumption and OH- generation. Alternatively, the reactive OH• radical may interact with lysosomal membrane lipids or proteins and disturb a normal transmembrane-dependent pH gradient [4,5]. Eventually, the elevated pH may then act as a negative feedback control to down regulate the activation of the NADPH oxidase and inhibit oxidative activity. Finally, generation of ROS during phagocytosis has been implicated in inactivation of lysosomal enzymes [13,14]. It is conceivable that an elevation of lysosomal pH to as high as 6.5–7.5 will inactivate various proteases in this organelle.
Figure 11.
Proposed mode of OxyBURST Green H2HFF BSA oxidation and the regulation of pH in lysosomes upon stimulation with phorbol ester.
Another novel finding in the present study is the heterogeneous distribution of oxidative activity and pH in lysosomal population responding to PMA treatment. Heterogeneous distribution of low molecular weight redox-active iron has been reported in lysosomal population within cells, which may contribute to differing stability of lysosomal membranes to oxidative stress as shown by Brunk and colleagues [15]. Although the regulation of this heterogeneity is too complicated and beyond the scope of the present study, it does open up another window for investigating lysosomal trafficking in various diseases. For example, the accumulation of lipids in lysosomal storage diseases appears to be heterogeneous [unpublished data]. Oxidative activity and pH distribution may be used as in situ indexes to improve our understanding of lipid accumulation in subsets of lysosomes. Understanding the regulation mechanism will be helpful in developing new treatment strategy for these diseases.
Conclusions
Using a novel indicator for oxidative activity, OxyBURST Green H2HFF BSA, I am able to study the distribution of lysosomal oxidative burst activity and its concurrent pH changes in live macrophage-like J744A.1 cell. The present result indicates that oxidative activity and pH in lysosomes are inter-regulated. Higher oxidative activity results in higher alkalinity while lower lysosomal pH is associated with lower oxidative activity. This approach provides a better opportunity to study the dynamic changes of oxidation and pH in phagolysosomes during phagocytosis, which can not be resolved by conventional biochemical analyses.
Methods
Reagents and cell cultures
OxyBURST Green H2HFF BSA, dextrans (10 K, lysine fixable) conjugated with either Cascade Blue or fluorescein fluorophores, LysoTracker Red DND-99 and 5-(and -6)-carboxyfluorescein were from Molecular Probes, Inc. (Eugene, OR). Nigericin, Monensin, Phorbol 12-myristate 13-acetate (PMA), horseradish peroxidase (HRP, type IV) and hydrogen peroxide (H2O2) were purchased from Sigma (St. Louis, MO). Potassium superoxide (KO2) was from Aldrich (Milwaukee, WI). All buffered solutions and other reagents for organic synthesis were analytical grade and freshly prepared.
Murine macrophage-like cell line (J774A.1) was from American Type Culture Collection (Manassas, VA). They were routinely grown in a monolayer (30%-50% confluence) on a glass cover slide at 37°C in DMEM (Dulbecco's Modified Eagle Medium), supplemented with 1% L-glutamine, 1% HEPES [4-(2-hydroxyethyl)piperazine-1-(2-ethanesulfonic acid)], 0.5% gentamicin, 1% penicillin/streptomycin and 10% Fetal Bovine Serum (Gibco, NY).
Measurement of oxidative activity in lysosomes using OxyBURST Green H2HFF BSA
The lysosomal population was first labeled with a Cascade Blue-conjugated dextran as previously described [3]. The cell monolayer was then incubated with 200 μg/mL OxyBURST Green H2HFF BSA in complete culture medium at 37°C for 2 hours. Afterwards, excess dye was removed by washing with phosphate-buffered saline (PBS) and the cells were incubated in complete culture medium in the presence or absence of 200 nM PMA at 37°C for an additional 0.5 hour. The cell monolayer was then washed with PBS and the fluorescent staining of the oxidized OxyBURST Green H2HFF BSA and Cascade Blue-conjugated dextran were then recorded by low-light level fluorescence microscopy with appropriate filters. The fluorescent intensity of the oxidized OxyBURST in the lysosomes was then quantified using the staining of Cascade Blue-conjugated dextran as a mask, as described previously [8]. The average fluorescence intensity (gray level/pixel area) in the mask region was then calculated and represents the relative oxidative activity in lysosomes. This procedure was repeated on images collected from seven or eight different cells in the same culture dish.
Measurement of Lysosomal pH
The pH of the lysosomal population in the J774A.1 cells was measured by a dual excitation ratiometric measurement according to established procedures using the fluorescein-conjugated dextran [3,8]. Namely, cells were incubated with 2–5 mg/mL fluorescein-conjugated dextran for approximately 18 hours in culture medium. Cells were washed and then incubated for an additional 0.5 hour in fresh culture medium in the presence or absence of 200 nM PMA. Samples were then washed with cold (4°C) MES (4-morpholineethanesulfonic acid) buffer solutions containing 5 mM NaCl, 115 mM KCl, 1.2 mM MgSO4 and 25 mM MES twice, and then observed under fluorescence microscope. Two low light level images of each cell were obtained at green emission region (λem = 515–565 nm), under sequential excitation by first at λex 485 nm, and then at λex 440 nm (10-nm band width). Background fluorescence was corrected by images of blank region of the same culture dish at both excitation wavelengths.
Imaging analysis to calculate the ratio of green fluorescence in lysosomes under excitation by 485 and 440 nm (" [F535(λ485)/F535 (λ440)]") was carried out as previously described [3]. The ratiometric data were converted to pH using a calibration curve from the lysosomes of ionophore-clamped cells. These cells were treated with 10 μM Monensin and 10 μM Nigericine and equilibrated for 2 min with MES (4-morpholineethanesulfonic acid) calibration buffer with pHs from 4.0 to 7.0 prior to image acquisition.
Selective staining of acidic lysosomes
In order to stain acidic lysosomes, LysoTracker Red DND-99 was used. The LysoTracker Red DND-99 is a fluorescent analog of chloroquine, which selectively accumulates in lysosomes [9]. Cells were incubated with a 25 nM concentration of the dye at 37°C for 5 min. Afterwards, excess dye was removed by washing with PBS. The fluorescence distribution was then viewed using a rhodamine filter.
Fluorescence microscopy and image processing
Fluorescence microscopy was performed with an inverted microscope (DIAPHOT-TMD, Nikon) equipped with a Plan Apo 60X (1.4 N.A.) objective and epifluorescence optics for various fluorophores including fluorescein (also for OxyBURST), Cascade Blue and rhodamine. A CCD camera (Quantix, Photometrics Ltd., AR) was used to obtain the microscopic images. Thirty-two fluorescence video images of cells were digitized (16 bits) and averaged using the Metamorph Image Processing system (Universal Imaging Corp., Media, PA).
Spectrofluorimetric analyses
Stock solutions of OxyBURST Green H2HFF BSA were prepared in phosphate buffered saline (PBS) and stored under nitrogen at 4°C. The HRP-catalyzed oxidation of this compound was determined spectroscopically as follows. To a PBS solution containing 50 μg/mL of OxyBURST Green H2HFF BSA, HRP was added to a final concentration of 4 μg/mL. The resulting solution was incubated at room temperature for 5 minutes to consume any endogenous H2O2. Afterwards, H2O2 was added, with stirring, to a final concentration of 10 nM. The spectral change in emission resulting from the oxidation of this compound was then recorded on a spectrophotometer (F-4500, Hitachi Instruments, Inc., Tokyo, Japan).
To study the effect of pH on the fluorescence of oxidized OxyBURST Green H2HFF BSA and 5-(and -6)-carboxyfluorescein, 78 μg/mL of the oxidized dye or 4.2 μM 5-(and -6)-carboxyfluorescein were prepared in MES (4-morpholineethanesulfonic acid) buffer solutions containing 5 mM NaCl, 115 mM KCl, 1.2 mM MgSO4 and 25 mM MES (pHs from 4.5 to 7.5). The fluorescence emission ratio at λ535 by excitation with λ485 over that by excitation with λ440 was then measured (i.e. F535(λ485)/F535 (λ440)). The effect of pH on the fluorescence of LysoTracker Red DND-99 (final concentration is 2 μM) was similarly performed, except that the emission fluorescence at λ592 was recorded upon excitation at λ574.
The effects of H2O2 and O2•- on the fluorescence of LysoTracker Red DND-99 were studied by preparing 2 μM solutions of the dye in MES buffer solutions at pH 4.5 or 7.5, containing various concentrations of H2O2 and KO2. After incubation at room temperature for 10 min, the emission fluorescence at λ592 was recorded as previously described.
The effect of oxidation on pH-dependency of fluorescein-conjugated dextran was tested in vitro by the HRP/H2O2 oxidant-generating system. Briefly, fluorescein dextran (final concentration at 30 μg/ml) was prepared in MES calibration buffers with pHs ranging from 3.0 to 7.3. These solutions were then incubated at 37°C for 30 min, in the absence or presence of H2O2 and HRP (final concentrations are 1 μM and 4 μg/mL, respectively). The fluorescence emission ratio, F535(λ485)/F535 (λ440), was then measured using SPEX Fluorolog 3 (Jobin Yvon Inc., Edison, NJ).
References
- Storrie B, Desjardins M. The biogenesis of lysosomes: is it a kiss and run, continuous fusion and fission process? BioEssays. 1996;18:895–903. doi: 10.1002/bies.950181108. [DOI] [PubMed] [Google Scholar]
- Brabowski GA, Gatt S, Horowitz M. Acid β-glucosidase: enzymology and molecular biology of Gaucher disease. Crit Rev Biochem Mol Biol. 1990;25:385–414. doi: 10.3109/10409239009090616. [DOI] [PubMed] [Google Scholar]
- Bach G, Chen CS, Pagano RE. Elevated lysosomal pH in Mucolipidosis type IV cells. Clin Chim Acta. 1999;280:173–179. doi: 10.1016/S0009-8981(98)00183-1. [DOI] [PubMed] [Google Scholar]
- Brunk UT, Cadenas E. The potential intermediate role of lysosomes in oxygen free radical pathology. APMIS. 1988;96:3–13. doi: 10.1111/j.1699-0463.1988.tb05261.x. [DOI] [PubMed] [Google Scholar]
- Zdolsek JM, Svensson I. Effect of reactive oxygen species on lysosomal membrane integrity. A study on a lysosomal fraction. Virchows Arch B Cell Pathol Incl ol Pathol. 1993;64:401–406. doi: 10.1007/BF02915141. [DOI] [PubMed] [Google Scholar]
- Aviram M, Rosenblat M, Etzioni A, Levy R. Activation of NADPH oxidase is required for macrophage-mediated oxidation of low-density lipoprotein. Metabolism. 1996;45:1069–1079. doi: 10.1016/s0026-0495(96)90005-0. [DOI] [PubMed] [Google Scholar]
- Robinson JP. Oxygen and nitrogen reactive metabolites and phagocytic cells. In: J. P. Robinson, G. F. Babcock, editor. Phagocyte Function: A Guide for Research and Clinical Evaluation. Wiley-Liss, Inc. New York.; 1998. pp. 217–252. [Google Scholar]
- Chen CS, Bach G, Pagano RE. Abnormal transport along the lysosomal pathway in Mucolipidosis, type IV disease. Proc Natl Acad Sci USA. 1998;95:6373–6378. doi: 10.1073/pnas.95.11.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wubbolts R, Fernandez-Borja M, Oomen L, Verwoerd D, Janssen H, Calafat J, Tulp A, Dusseljee S, Neefjes J. Direct vesicular transport of MHC class II molecules from lysosomal structures to the cell surface. J Cell Biol. 1996;135:611–22. doi: 10.1083/jcb.135.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer SJ, Keen PM, Craven N, Bourne FJ. Regulation of phagolysosome pH in bovine and human neutrophils: the role of NADPH oxidase activity and an Na+/H+ antiporter. J Leukoc Biol. 1989;45:239–248. doi: 10.1002/jlb.45.3.239. [DOI] [PubMed] [Google Scholar]
- Ryan TC, Weil GJ, Newburger PE, Haugland R, Simons ER. Measurement of superoxide release in the phagovacuoles of immune complex-stimulated human neutrophils. J Immunol Methods. 1990;130:223–233. doi: 10.1016/0022-1759(90)90052-W. [DOI] [PubMed] [Google Scholar]
- Styrt B, Klempner MS. Inhibition of neutrophil oxidative metabolism by lysosomotropic weak bases. Blood. 1986;67:334–342. [PubMed] [Google Scholar]
- Kindzelskii AL, Zhou MJ, Haugland RP, Boxer LA, Petty HR. Oscillatory pericellular proteolysis and oxidant deposition during neutrophil locomotion. Biophys J. 1998;74:90–97. doi: 10.1016/S0006-3495(98)77770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voetman AA, Weening RS, Hamers MN, Meerhof LJ, Bot AA, Roos D. Phagocytosing human neutrophils inactivate their own granular enzymes. J Clin Invest. 1981;67:1541–1549. doi: 10.1172/JCI110185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E, Ghassemifar R, Brunk UT. Lysosomal heterogeneity between and within cells with respect to resistance against oxidative stress. Histochem J. 1997;29:857–865. doi: 10.1023/A:1026441907803. [DOI] [PubMed] [Google Scholar]