Abstract
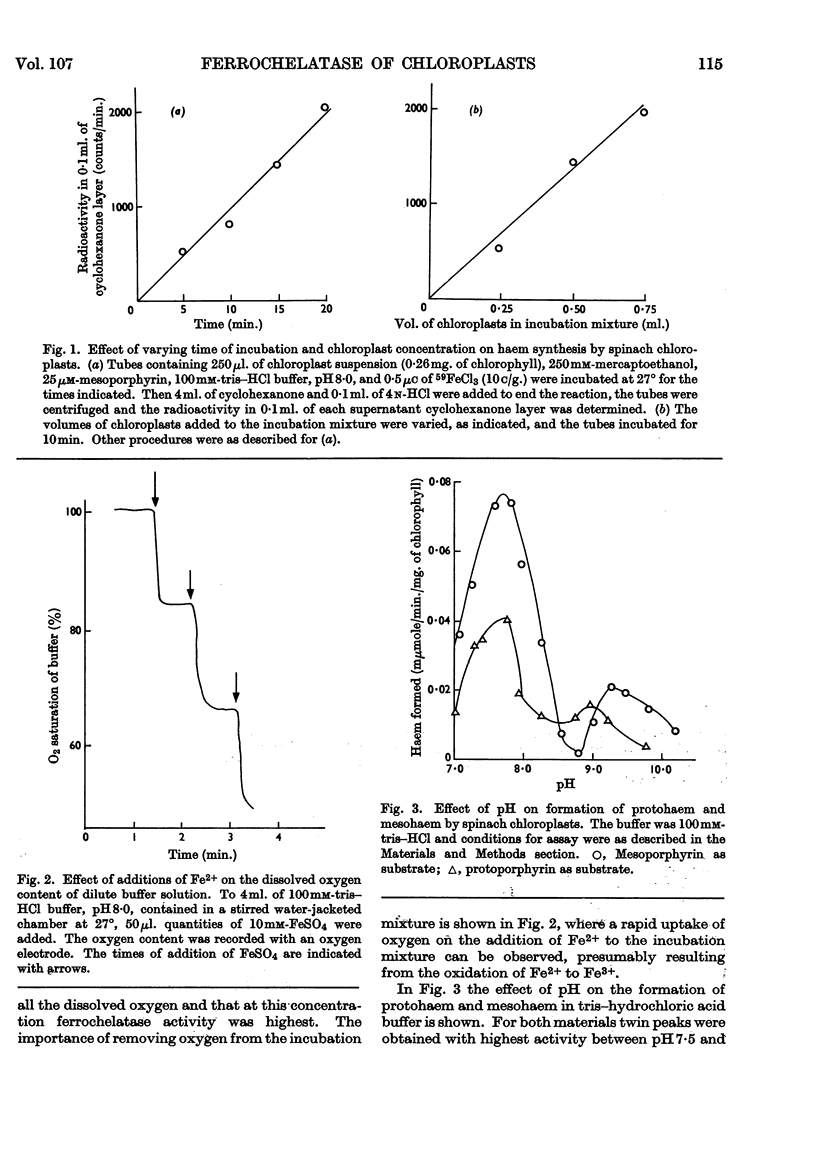
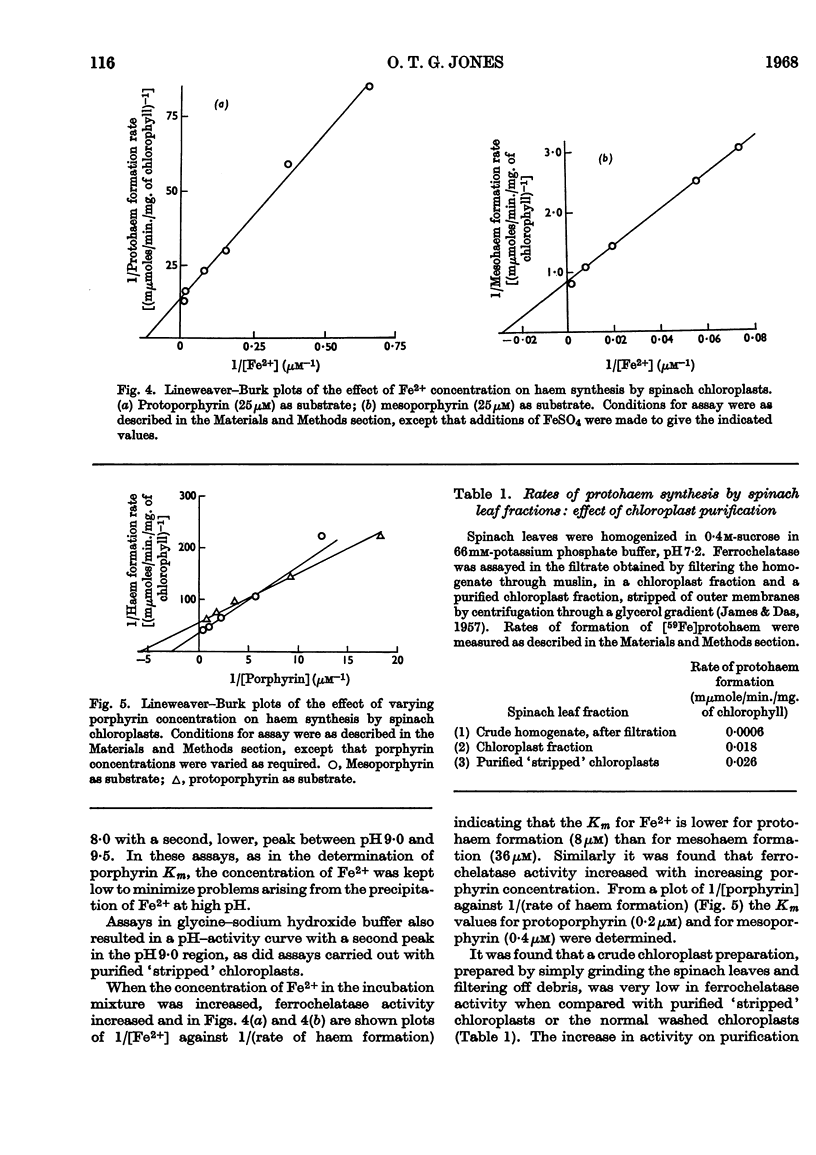
Spinach chloroplasts catalyse the incorporation of Fe2+ into protoporphyrin, mesoporphyrin and deuteroporphyrin to form the corresponding haems. This ferrochelatase activity was detected by pyridine haemochrome formation with acetone-dried powders of chloroplasts, or from the formation of [59Fe]haems by intact chloroplasts. Decreasing the mitochondrial contamination of the chloroplasts by density-gradient centrifugation did not cause any loss of activity: spinach ferrochelatase appears to be principally a chloroplast enzyme. The characteristics of the enzyme were examined by using [59Fe]haem assay. The activity was pH-dependent: for both mesohaem and protohaem formation there were two pH maxima, a major peak at about pH7·8 and a smaller peak at about pH9·2. Lineweaver–Burk plots showed that the Km for Fe2+ incorporation into protoporphyrin was 8μm and that for Fe2+ incorporation into mesoporphyrin was 36μm. At non-saturating Fe2+ concentrations the Km for protoporphyrin was 0·2μm and that for mesoporphyrin was 0·4μm. Ferrochelatase was not solubilized by treatment of chloroplasts with ultrasound but was solubilized by stirring in 1% (w/v) Tween 20 at pH10·4. Unlike the rat liver mitochondrial enzyme, chloroplast ferrochelatase was not stimulated by treatment with selected organic solvents. The spinach enzyme was inactive in aerobic conditions and it was shown by using an oxygen electrode that under such conditions the addition of Fe2+ to buffer solutions caused a rapid uptake of dissolved oxygen, believed to be due to the oxidation of Fe2+ to Fe3+; Fe3+ is not a substrate for ferrochelatase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- JOHNSON A., JONES O. G. ENZYMIC FORMATION OF HAEMS AND OTHER METALLOPORPHYRINS. Biochim Biophys Acta. 1964 Oct 9;93:171–173. doi: 10.1016/0304-4165(64)90273-9. [DOI] [PubMed] [Google Scholar]
- KRANTZ S. B., GALLIEN-LARTIGUE O., GOLDWASSER E. THE EFFECT OF ERYTHROPOIETIN UPON HEME SYNTHESIS BY MARROW CELLS IN VITRO. J Biol Chem. 1963 Dec;238:4085–4090. [PubMed] [Google Scholar]
- LABBE R. F., HUBBARD N. Metal specificity of the ironprotoporphyrin chelating enzyme from rat liver. Biochim Biophys Acta. 1961 Sep 2;52:130–135. doi: 10.1016/0006-3002(61)90910-6. [DOI] [PubMed] [Google Scholar]
- LABBE R. F., NISHIDA G. A new method of hemin isolation. Biochim Biophys Acta. 1957 Nov;26(2):437–437. doi: 10.1016/0006-3002(57)90033-1. [DOI] [PubMed] [Google Scholar]
- Mazanowska A. M., Neuberger A., Tait G. H. Effect of lipids and organic solvents on the enzymic formation of zinc protoporphyrin and haem. Biochem J. 1966 Jan;98(1):117–127. doi: 10.1042/bj0980117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIDA G., LABBE R. F. Heme biosynthesis; on the incorporation of iron into protoporphyrin. Biochim Biophys Acta. 1959 Feb;31(2):519–524. doi: 10.1016/0006-3002(59)90028-9. [DOI] [PubMed] [Google Scholar]
- Nandi D. L., Waygood E. R. Biosynthesis of porphyrins in wheat leaves. I. Succinate:coA ligase (ADP). Can J Biochem. 1965 Oct;43(10):1605–1614. doi: 10.1139/o65-177. [DOI] [PubMed] [Google Scholar]
- Neuberger A., Tait G. H. Studies on the biosynthesis of porphyrin and bacteriochlorophyll by Rhodopseudomonas spheroides. 5. Zinc-protoporphyrin chelatase. Biochem J. 1964 Mar;90(3):607–616. doi: 10.1042/bj0900607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 1. Assay and properties of ferrochelatase from a pig-liver mitochondrial extract. Biochem J. 1963 Apr;87:181–185. doi: 10.1042/bj0870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 2. An in vestigation of the role offerrochelatase in the biosynthesis of various haem prosthetic groups. Biochem J. 1963 Apr;87:186–192. doi: 10.1042/bj0870186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. N. Some aspects of metal incorporation into porphyrins. Enzymologia. 1967 Jan 31;32(1):13–17. [PubMed] [Google Scholar]
- Porra R. J., Vitols K. S., Labbe R. F., Newton N. A. Studies on ferrochelatase. The effects of thiols and other factors on the determination of activity. Biochem J. 1967 Aug;104(2):321–327. doi: 10.1042/bj1040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]
- Yoneyama Y., Tamai A., Yasuda T., Yoshikawa H. Iron-chelating enzyme from rat liver. Biochim Biophys Acta. 1965 Jul 29;105(1):100–105. doi: 10.1016/s0926-6593(65)80178-3. [DOI] [PubMed] [Google Scholar]



