Abstract
A total of 14 lambs were experimentally infected with Eimeria ovinoidalis in two separate experiments in two consecutive years. Nine lambs served as uninoculated controls. Material was collected from the ileum 2 weeks after infection in eight lambs and 3 weeks after infection in six lambs. Lambs examined 2 weeks after infection had normal follicles. After three weeks, the follicle-associated epithelium covering the lymphoid follicles of the ileal Peyer’s patches showed fusions with adjacent absorptive epithelium, focal hyperplasia, and occasionally necrosis. Macrogametes, microgamonts, and oocysts were often found in the follicle-associated epithelium and the dome region. Various degrees of lymphocyte depletion were present in the ileal lymphoid follicles in all six infected lambs 3 weeks after infection, and four lambs had decreased follicle size. Reduced staining for leukocyte common antigen (CD45), B-cell markers, and the proliferation marker Ki-67 was present in these lambs. Application of the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling method for apoptotic cells revealed decreased staining in the ileal lymphoid follicles 3 weeks after infection. A marker of follicular dendritic cells, 5′- nucleotidase, showed increased reactivity, probably due to condensation of reticular cells following loss of follicle lymphocytes. Reduced staining for carbonic anhydrase in the follicle-associated epithelium and the domes was present in all six lambs examined 3 weeks after infection, indicating decreased production of carbonic anhydrase-reactive 50-nm particles and a decreased lymphoproliferative stimulus. In conclusion, the present study shows that severe E. ovinoidalis infection in lambs causes lesions of the follicle-associated epithelium and may result in lymphocyte depletion and atrophy of the ileal Peyer’s patch follicles.
Coccidiosis in lambs is an intestinal infection characterized clinically by diarrhea and dehydration. A major cause of ovine coccidiosis is Eimeria ovinoidalis, but other species, such as Eimeria crandallis, may also be associated with this disease. Morphological changes in Eimeria sp. infections in lambs are characterized by inflammation, villus atrophy, epithelial hyperplasia, glandular loss, and mucosal collapse (8, 9, 11). Cellular immune responses play an important role in immunity against Eimeria spp., whereas humoral immunity probably represents a less important factor (3, 46, 51).
Immunity is dependent on effective antigen detection, and the Peyer’s patches are important for the generation of an immune response. Ruminants have two distinct types of Peyer’s patches, the jejunal Peyer’s patches and the ileal Peyer’s patches (IPP), which differ in their development, cellular composition, and function (13, 28, 45). IPP have been attributed to have a role as a central lymphoid organ in the development of B cells, whereas a function in mucosal immune responses has been questioned (12, 36). The Peyer’s patches of ruminants are outlined by a modified, follicle-associated epithelium (FAE), which has the ability to internalize and transport antigens to the underlying lymphoid tissue (22). In ruminants, the FAE of the IPP and that of the jejunal Peyer’s patches differ in morphology and enzyme reactivity (21, 22). Whereas the jejunal FAE is composed of absorptive epithelial cells intermingled with modified membranous cells, M cells, the ileal FAE consists of a homogenous population of cells characterized by active transcytosis of macromolecules and shedding of carbonic anhydrase-reactive 50-nm membrane-bound particles to the underlying lymphoid tissue (22).
The FAE has the capacity for uptake of microorganisms (reviewed in references 37 and 47) and may provide a route of entry for multiple bacterial and viral pathogens (5, 15, 16, 23, 24, 32, 48, 51). The uptake of pathogens by the FAE may be followed by injury to the lymphoid follicles. Such lesions can vary from lymphocyte depletion to extensive destruction of lymphoid follicles (2, 19, 26, 29, 52, 53).
The aims of the present study were to examine lesions of the FAE and the IPP follicles during infection with an enteric protozoan species in lambs. Changes in morphology and enzyme reactivity in the FAE and the lymphoid follicles were examined. Alterations in cellular composition and the rate of proliferation and apoptosis in the lymphoid follicles of the IPP were addressed.
MATERIALS AND METHODS
Experimental design and animals.
A total of 23 lambs were used; 14 were infected with sporulated oocysts of E. ovinoidalis, and 9 served as uninoculated controls. Lambs were obtained from the Dal Research Farm, Asker, Norway, and were of the Dala breed. The lambs were used in two separate experiments, and the experimental protocol has been described in detail elsewhere (1). In experiment 1, eight lambs (no. 1 to 8) were infected when 19 to 21 days old and euthanatized 10 (one lamb) or 13 days after infection. Each lamb was given 250,000 oocysts. Five lambs (no. 9 to 13) served as uninoculated controls and were killed when they were 30 days old. In experiment 2, six lambs (no. 14 to 19) were each given 100,000 oocysts at the age of 19 to 21 days and euthanatized 19 (one lamb) or 20 days after infection. Four lambs (no. 20 to 23) served as uninoculated controls in experiment 2 and were killed when they were 39 or 40 days old. The inoculum contained oocysts of the same isolate of E. ovinoidalis (>99.9% pure) in both experiments.
Clinical examination.
A thorough clinical examination of the lambs was not performed, but clinical signs were briefly recorded at the time of euthanasia.
Collection of tissue specimens.
Pieces of tissue were collected under pentobarbital anesthesia (20 mg/kg of body weight) or were taken promptly at necropsy following euthanasia with pentobarbital. For the present study, specimens were taken from the IPP at the insertion of the ileocecal ligament. For morphometric analysis, an additional sample was taken from the IPP close to the ileocecal valve. Replicate samples were taken; one specimen was frozen in chlorodifluoromethane (Isceon 22), and the other was fixed in 10% phosphate-buffered formalin. Frozen samples were stored at −70°C until tested.
Histopathological examination.
Fixed sections were routinely processed and embedded in paraffin. Sections were cut 2 to 5 μm thick and stained with Mayer’s hematoxylin and eosin. Stained sections were examined by light microscopy.
A semiquantitative evaluation of the cellularity in the IPP follicles was done independently by two persons. The slides were read in a blinded fashion by use of a four-point (0 to +++) scoring system for examination of lymphocyte depletion. The average value for each individual was calculated. The results were transformed to numeric values, and differences in values between infected and control lambs were tested by using the Mann-Whitney test. The level of significance chosen was a P value of <0.05.
5′-Nucleotidase histochemical analysis.
Frozen samples were cut to a thickness of 7 μm, and the sections were air dried at room temperature for 1 to 2 h and fixed for 5 min at 4°C in 4% formaldehyde containing 68 mM calcium chloride adjusted to pH 7.2 with sodium hydroxide. Reactivity for 5′-nucleotidase was examined by incubation with a solution composed of 1 mM AMP, 200 mM Trismaleate buffer (pH 7.4), 1.3 mM lead nitrate, 100 mM MgSO4 · 7H2O, and 96 mM sucrose (35). The sections were incubated for 40 min at 37°C, followed by treatment with a 0.2% (NH4)2S solution for 1 min. The addition of 100 mM sodium fluoride to the incubation solution inhibited the reaction.
Carbonic anhydrase histochemical analysis.
Frozen samples were cut and the sections were fixed as described for the 5′-nucleotidase analysis. Carbonic anhydrase was demonstrated by a modification of the method of Ridderstrale (45). The incubation medium contained 3.5 mM CoSO4, 26 mM H2SO4, 12 mM KH2PO4, and 157 mM NaHCO3. The total incubation time was 3 or 4 min and involved repeated dipping of the sections into the incubation medium for 2 s and withdrawal for 20 s. The sections were then rinsed for 5 min in 0.7 mM phosphate buffer (pH 5.9) before treatment with a freshly prepared 0.2% (NH4)2S solution for 1 min. Finally, the sections were washed in water before mounting. Controls included specific inhibition by the addition of 10−5 mM acetazolamide to the incubation medium.
Staining for apoptotic cells.
An in situ cell death detection kit (Boehringer Mannheim GmbH, Mannheim, Germany) based on the TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) method was used to demonstrate apoptotic cells (6). Sections 7 μm thick were made from frozen samples and fixed for 20 min in 4% paraformaldehyde at room temperature. The kit was used as recommended by the manufacturer. DNA strand breaks are produced during apoptosis and are identified by labeling free 3′-OH ends with modified (fluorescein isothiocyanate-labeled) nucleotides in an enzymatic reaction. Terminal deoxynucleotidyltransferase is the enzyme used in this kit. Incorporated fluorescein is detected by use of a sheep antifluorescein antibody, conjugated with alkaline phosphatase, followed by treatment with fast red.
Immunohistochemical analysis.
An avidin-biotin complex method was used for immunoperoxidase staining. Cryostat sections were cut to a thickness of about 7 μm. The sections were air dried at room temperature for 1 h and fixed in acetone for 10 min. After fixation, the sections were air dried for 5 min, briefly warmed with a hair dryer, and rehydrated in phosphate-buffered saline. Fixed sections were treated with normal horse or goat serum diluted 1:50 in 5% bovine serum albumin (BSA) in Tris-buffered saline (TBS) containing avidin solution (from an avidin-biotin blocking kit; Vector Laboratories, Burlingame, Calif.) diluted 1:6 for 20 min at room temperature. All incubations were done in a slowly rotating humid chamber, and washing between each step was done with phosphate-buffered saline. The primary antibodies (Table 1) were diluted in 1% BSA-TBS containing biotin solution (from the avidin-biotin blocking kit) diluted 1:6, applied to the sections, and incubated overnight at 4°C. Biotinylated horse anti-mouse immunoglobulin G or anti-rabbit immunoglobulin G (Vector Laboratories) was diluted 1:200 in 1% BSA-TBS containing 2% normal sheep serum and incubated with the sections for 30 min at room temperature. Subsequently, inhibition of endogenous peroxidase was carried out by treatment with 0.03% H2O2 in 50 mM imidazole buffer (pH 7.4) for 10 min. Avidin-biotin-horseradish peroxidase complex (Vector Laboratories) was diluted 1:200 in phosphate-buffered saline and incubated for 30 min. Peroxidase reactivity was visualized by treatment for 2 min with a metal-enhanced diaminobenzidine substrate (Pierce Chemical Company, Rockford, Ill.) diluted 1:10 in peroxide substrate buffer. Sections were rinsed in distilled water and stained with Mayer’s hematoxylin for 1 min before mounting.
TABLE 1.
Antibodies to leukocyte molecules used in this study
| Mole-cule | Antibodies | Cells or antigen marked | Refer-ence |
|---|---|---|---|
| CD45 | SBU-LCA | Leukocyte common antigen | 30 |
| CD21 | DU2-74-25 | B cells, follicular den-dritic cells | 14 |
| BLA.36 | Mouse anti-human B lympho-cyte BLA.36 | B cells | 17 |
| Ki-67 | Mouse anti-human Ki-67 antigen | Proliferating cells | 7 |
| CD68 | Mouse anti-human macro-phage CD68 | Macrophages | 18 |
Morphometric analysis.
In all animals from both experiments, the sizes of the ileal follicles were examined by morphometric analysis. From both sites sampled, two sections of frozen tissue at a distance of 175 μm were made. Tissue blocks were oriented prior to sectioning in order to cut the majority of the lymphoid follicles longitudinally. Sections were stained for 5′-nucleotidase as described above and then counterstained with eosin for 1 min. Images of all follicles identified by light microscopic examination were captured using a CCD-72 video camera (Dage-MTI Inc., Michigan City, Ind.) mounted on a Leitz Orthoplan microscope and Image Grabber software (Neotech Ltd., Hampshire, England). The mean follicle size for each animal was estimated using a video image analysis system (analySIS; Soft-Imaging Software GmbH, Münster, Germany). All follicular lymphoid tissue below the muscularis mucosae was measured. Follicles with a disrupted fibrous capsule were excluded. A mean value was calculated for the two sites sampled. The Mann-Whitney test was used to test for differences in mean follicle sizes between infected and control animals. The level of significance chosen was a P value of <0.05.
RESULTS
Clinical signs.
Infected lambs in experiment 1 were all healthy, except for mild diarrhea in lamb no. 2 and 3. In experiment 2, lamb no. 16 showed hemorrhagic diarrhea and severe dehydration, and this lamb was moribund 21 days after infection. Lamb no. 15 also showed severe dehydration and enteritis. The other infected lambs in experiment 2 showed diarrhea and moderate dehydration 21 days after infection.
Morphological changes.
Histopathological findings are summarized in Table 2. Lambs in experiment 1 had mild lesions of the FAE. Five animals had epithelial adhesions, whereas mild and focal epithelial hyperplasia was observed in three lambs. Developmental stages of coccidia, mainly second-generation meronts and gamonts, were occasionally seen in the FAE. The crypts surrounding the dome were often dilated and contained debris and neutrophils. The lymphoid follicles appeared normal in infected lambs in experiment 1, and semiquantitative evaluation of lymphocyte density in IPP follicles did not show significant differences between infected and control lambs.
TABLE 2.
Evaluation of morphological changes and carbonic anhydrase reactivity in IPP of lambs infected with E. ovinoidalis
| Lamb | Morphological changesa in the ileal dome or follicle region
|
Carbonic anhydrase reactivityb in:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Epithelial adhesions | Intra-epithelial parasites | FAE hyper-plasia | Dome atrophy | Lympho-cyte de-pletion | FAE | Dome area | Follicle center | |
| Controls | (+) | 0 | 0 | 0 | 0 | +++ | +++ | +++ |
| 1 | ++ | 0 | 0 | 0 | 0 | +++ | +++ | +++ |
| 2 | ++ | (+) | 0 | 0 | 0 | +++ | +++ | +++ |
| 3 | ++ | ++ | + | 0 | 0 | ++ | ++ | ++ |
| 4 | 0 | 0 | 0 | 0 | 0 | +++ | +++ | +++ |
| 5 | 0 | 0 | 0 | 0 | 0 | +++ | +++ | +++ |
| 6 | 0 | 0 | 0 | (+) | 0 | ++ | ++ | ++ |
| 7 | + | + | + | (+) | 0 | + | ++ | +++ |
| 8 | + | ++ | + | 0 | 0 | ++ | ++ | +++ |
| 14 | +++ | +++ | ++ | ++ | ++ | + | + | ++ |
| 15c | ++ | +++ | + | ++ | + | + | + | +++ |
| 16 | +++ | ++ | +++ | +++ | +++ | + | + | + |
| 17c | ++ | + | 0 | ++ | ++ | + | + | ++ |
| 18 | +++ | +++ | +++ | ++ | + | + | + | ++ |
| 19 | +++ | ++ | + | +++ | + | + | + | ++ |
+++, prominent lesions; ++, moderate lesions; +, mild lesions; (+), few, scattered lesions; 0, no observed lesions.
+++, strong staining; ++, moderate staining; +, weak staining.
IPP of terminal ileum were evaluated.
Infected lambs in experiment 2 had severe lesions of the FAE (Table 2). Changes included extensive parasitization of epithelial cells, fusions with adjacent absorptive epithelium, and epithelial hyperplasia (Fig. 1 and 2). One lamb (no. 17) had extensive necrosis of the FAE, whereas four lambs had small necrotic foci in the FAE. The domes were small and inconspicuous in five lambs, and various coccidial stages were often present within the dome region (Fig. 2). All six infected lambs in experiment 2 had various degrees of lymphocyte depletion of the IPP follicles. Lamb no. 16 had severe lymphocyte depletion (Fig. 3), whereas loss of lymphocytes was moderate or mild in the other five lambs (Table 2). Statistical analysis of the data collected by semiquantitative evaluation showed significant differences in the cellularity of lymphoid follicles between infected and control animals (P < 0.05). Lamb no. 16 had distinct follicle atrophy, and three other lambs (no. 15, 18, and 19) had moderately reduced follicle size. The follicle capsule was thickened, and perifollicular lymphatic sinuses were dilated. Lamb no. 18 had some intrafollicular abscesses.
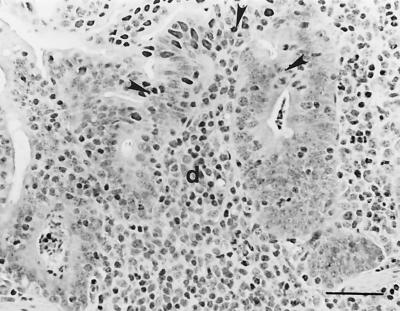
FIG. 1.
Dome (d) of IPP of a lamb (no. 14) infected with E. ovinoidalis. The FAE covering the dome shows fusions with adjacent absorptive epithelium (arrowheads). Hematoxylin-eosin. Bar, 50 μm.
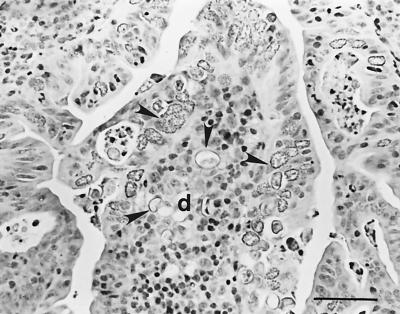
FIG. 2.
Additional aspects of the dome (d) of IPP of a lamb (no. 14) infected with E. ovinoidalis. Numerous macrogametes and oocysts are present in the FAE and in the dome region (arrowheads). Hematoxylin-eosin. Bar, 50 μm.
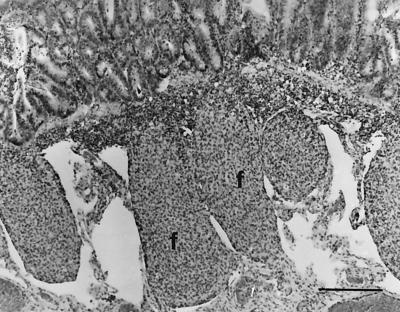
FIG. 3.
IPP of lamb no. 16 infected with E. ovinoidalis. The lymphoid follicles (f) are reduced in size and depleted of lymphocytes. Hematoxylin-eosin. Bar, 205 μm.
Morphometric analysis.
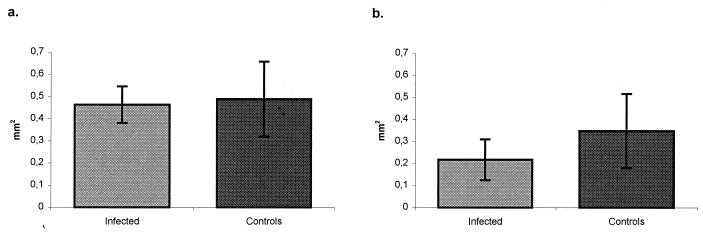
Data on mean follicle size in animals from experiments 1 and 2 are shown in Fig. 4a and b, respectively. Differences in follicle size between infected and control animals were not significant at the chosen level (P < 0.05).
FIG. 4.
Mean and standard error of the mean area of the follicles of the IPP of age-matched control lambs and infected lambs, as determined using computer-assisted morphometric analysis. (a) Infected lambs (no. 1 to 8) and control lambs (no. 9 to 13) killed 2 weeks after infection. (b) Infected lambs (no. 14 to 19) and control lambs (no. 20 to 23) killed 3 weeks after infection.
5′-Nucleotidase histochemical analysis.
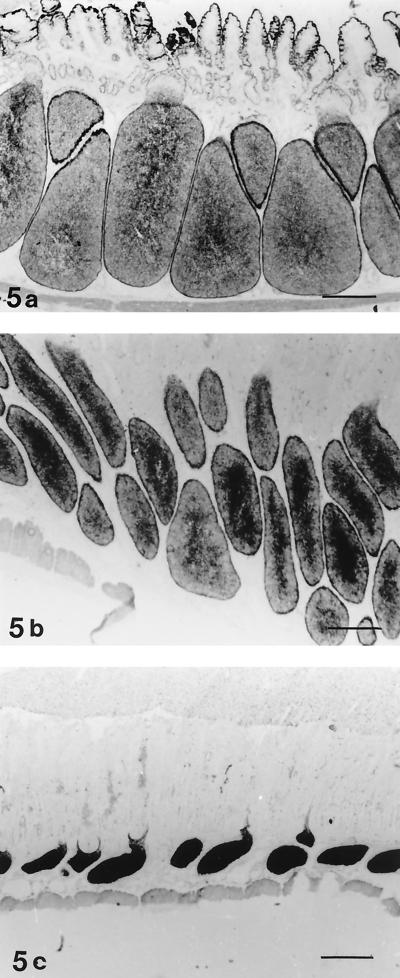
5′-Nucleotidase is a marker for follicular dendritic cells. In control lambs, the ileal lymphoid follicles showed reticular staining for 5′-nucleotidase (Fig. 5a). The reactivity for this enzyme in the ileal lymphoid follicles of infected lambs showed variation between individuals. All infected lambs in experiment 1 showed reticular staining like that in the controls. Increased reactivity for 5′-nucleotidase was observed in the follicle centers in the three lambs with moderate follicle atrophy (Fig. 5b). Lamb no. 16, with severe lymphocyte depletion and atrophy, had even, intense staining of the follicle centers, indicating condensation of stromal cells due to loss of lymphocytes (Fig. 5c).
FIG. 5.
5′-Nucleotidase staining of IPP. (a) Reticular staining in the ileal lymphoid follicles of a control lamb (no. 23). Bar, 440 μm. (b) Lamb no. 18 infected with E. ovinoidalis. Moderate follicle atrophy is present. The lymphoid follicles show reticular staining for 5′-nucleotidase in the peripheral zone, whereas the central zone in many follicles stains more intensely. Bar, 440 μm. (c) Coccidium-infected lamb (no. 16) with severe follicle atrophy. Even, intense staining due to condensation of stromal cells and lymphocyte depletion is present in the lymphoid follicles. Bar, 460 μm.
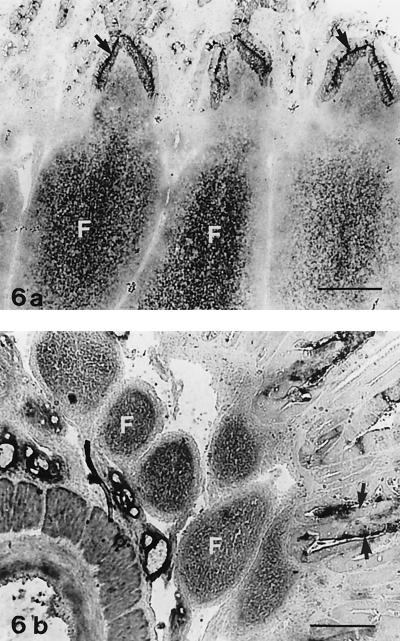
Carbonic anhydrase histochemical analysis.
Carbonic anhydrase is reported to show strong staining of the ileal FAE and of IPP follicle centers in ruminants (25). In accordance with these data, strong reactivity for carbonic anhydrase was found in the ileal FAE of control lambs in the present study; enzyme reactivity was located at the luminal cell border and along the basolateral plasma cell membrane (Fig. 6a), as described by Landsverk et al. (25). In normal ileal lymphoid follicles, granular staining for carbonic anhydrase was present in the follicle centers (Fig. 6a). Four infected lambs in experiment 1 had slightly reduced reactivity for carbonic anhydrase in the FAE and dome (Table 2), whereas the others showed reactivity like that in the controls. All six lambs euthanatized 3 weeks after infection showed reduced staining for carbonic anhydrase in the FAE and dome (Table 2). Reduced staining for carbonic anhydrase was also present in the follicle centers in five of the six lambs (Fig. 6b).
FIG. 6.
Carbonic anhydrase staining of the IPP. (a) Lymphoid follicles of a control lamb (no. 22). The FAE (arrows) shows reactivity along the apical and lateral plasma cell membranes. Note the granular staining of the follicle centers (F). Bar, 205 μm. (b) Coccidium-infected lamb (no. 16) with severe atrophy. The FAE (arrows) shows focal reactivity at the luminal cell membrane, whereas staining along the lateral cell membrane is absent or weak. The follicle centers (F) show diffuse, weak staining. Bar, 205 μm.
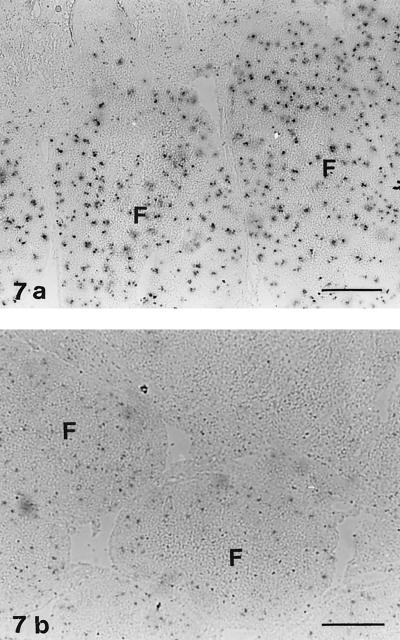
Apoptosis.
Ileal follicles in control lambs contained several apoptotic cells (Fig. 7a), a finding in accordance with the apoptosis of B lymphocytes that normally occurs in the IPP (34, 42, 44). Reduced staining for apoptotic cells was found in infected lambs (no. 15, 16, 18, and 19) with follicle atrophy (Fig. 7b).
FIG. 7.
Staining with the TUNEL method for apoptotic cells. (a) IPP of a control lamb (no. 22). Numerous apoptotic cells are present in the lymphoid follicles (F). Bar, 205 μm. (b) IPP of lamb no. 16 infected with E. ovinoidalis. Few apoptotic cells are present in the follicles (F). Bar, 102 μm.
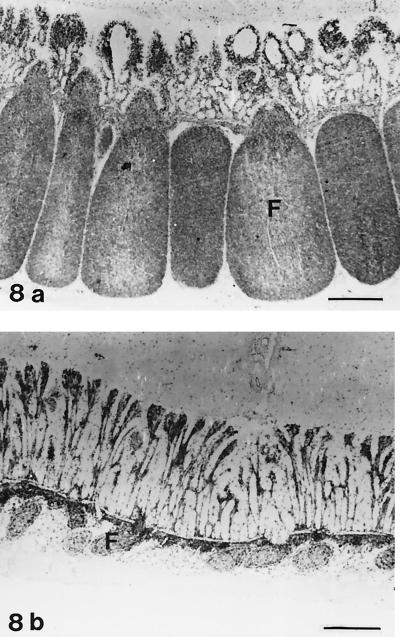
Immunohistochemical analysis.
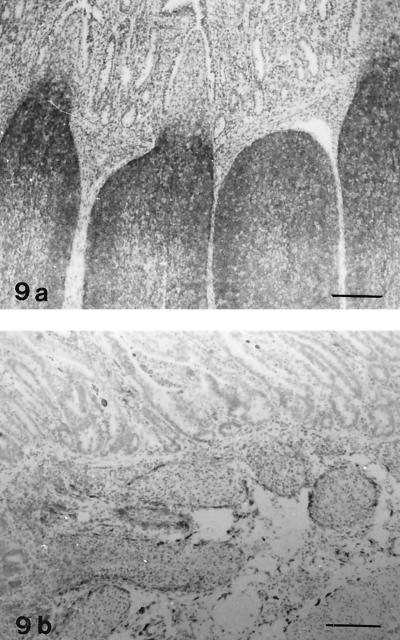
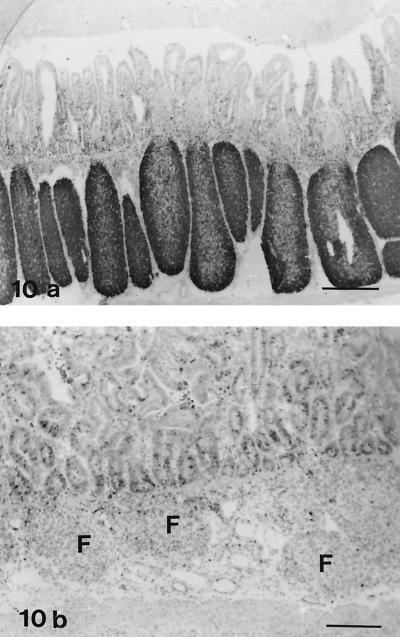
Follicles in lamb no. 16, showing severe lymphocyte depletion, contained only a few cells with distinct staining for leukocyte common antigen (CD45); in contrast, in control lambs most of the follicle lymphocytes were positive (Fig. 8). B cells, which showed positive staining with antibodies against CD21 and BLA.36, were numerous in normal lymphoid follicles (Fig. 9a), whereas only scattered cells were present in the atrophic follicles of lamb no. 16 (Fig. 9b). In the IPP follicles of the three lambs with moderate atrophy, reduced numbers of CD45+ cells and CD21+ cells were present. Staining for the nuclear antigen Ki-67, which is expressed on proliferating cells (7), was decreased in the IPP follicles of lamb no. 15 to 19 compared with normal lambs (Fig. 10). Macrophages were present in the lymphoid follicles of both infected and control lambs, as seen in sections stained for CD68. Condensed staining for this marker was present in atrophic follicles (data not shown).
FIG. 8.
IHC analysis for leukocyte common antigen (CD45). Avidin-biotin-peroxidase complex method. Hematoxylin counterstain. (a) IPP of a control lamb (no. 23). The majority of cells in the follicles (F) are CD45 positive. Bar, 480 μm. (b) IPP of lamb no. 16 infected with E. ovinoidalis. The atrophic ileal follicles (F) contain scattered CD45-positive cells. Bar, 490 μm.
FIG. 9.
IHC analysis for B-cell marker CD21. Avidin-biotin-peroxidase complex method. Hematoxylin counterstain. (a) IPP of a control lamb (no. 20). Numerous positive cells are present in the follicles. Bar, 175 μm. (b) IPP of an infected lamb (no. 16). B cells are scarce in the lymphoid follicles. Bar, 175 μm.
FIG. 10.
IHC analysis for proliferation marker Ki-67. Avidin-biotin-peroxidase complex method. Hematoxylin counterstain. (a) IPP of a control lamb (no. 20). Intense staining of follicle lymphocytes is present. Bar, 480 μm. (b) IPP of an infected lamb (no. 16). Few cells in the follicles (F) are positive. Prominent follicle atrophy is present. Bar, 480 μm.
DISCUSSION
The present study shows that patent infection with E. ovinoidalis may result in lymphocyte depletion and atrophy of IPP follicles in lambs. A significant reduction in the cellularity of IPP follicles was present in lambs 3 weeks after infection, a finding that may represent an initial step in the regression of follicles. Four infected lambs in experiment 2 had reduced follicle size, but statistical analysis of data obtained by computer-assisted morphometric examination did not show a significant difference in follicle size between infected and control lambs. One must keep in mind the facts that the numbers of animals in both groups in experiment 2 were very low and that considerable variation between individuals in each group was present. Still, we believe that reduced follicle size was a characteristic finding in four lambs 3 weeks after infection. Lymphocyte depletion of the Peyer’s patches in coccidiosis has been reported as a finding in severe disease in lambs (8, 9, 11) and rabbits (10). Our study shows that lesions present in the follicles varied between individuals given the same dose of oocysts, indicating that other aspects may be important. Factors such as genetic constitution, maternal immunity, and the immune status of the animal probably influence the severity of disease (8, 46) and may, at least in part, contribute to the variation observed between individuals.
Neither decreased cellularity nor reduced size was present in IPP follicles in lambs 2 weeks after infection. This finding implies that lymphocyte depletion of IPP follicles in lambs infected with E. ovinoidalis may develop between 14 and 21 days after inoculation. The prepatent period for E. ovinoidalis is approximately 15 days, and the present study shows that patent infection was present before lymphocyte depletion of IPP follicles occurred. However, various unknown factors also could have contributed to the differences in follicle morphology and size observed between the infected groups. First, the experiments were carried out in two consecutive years. Various environmental factors may have changed, but the housing facilities and feeding routines were the same in both experiments (1). The immune status of the lambs in the two experiments may have differed, even though the lambs were kept together with their mothers both years. The infective dose was reduced in experiment 2 in order to prevent serious, life-threatening enteritis. Still, more prominent lesions of the intestinal mucosa and the IPP were present in these lambs.
It is possible that injury to the FAE and dome precedes follicle atrophy and results in the decreased lymphocyte population of IPP follicles that is present 3 weeks after infection. Mild lesions of the FAE were observed in five of eight infected lambs 2 weeks after infection, and severe epithelial injury was found in all six infected lambs after 3 weeks. Hence, it is possible that lymphocyte depletion represents a sequela to damage to the FAE.
Normal FAE function is probably important for the maintenance of follicle lymphopoiesis (22, 27). The FAE is known to be active in the transcytosis of macromolecular antigens. However, it has been shown that lymphopoiesis in IPP follicles is largely independent of external antigens (40). In fact, IPP has been attributed a role as a central lymphoid organ rather than as a secondary one (reviewed by Griebel and Hein [12]). Landsverk (22) previously characterized an epithelial product that is produced independently of luminal antigen in the ileal FAE. This product consists of membrane-bound particles with a diameter of about 50 nm. They are produced in the ileal FAE and then secreted to lacunae located at the lateral cell wall. Secreted particles are transported to the follicle centers, where they adhere to follicle lymphocytes and are incorporated into their cytoplasm. A characteristic of the membrane-bound particles produced in the ileal FAE is their reactivity for carbonic anhydrase. In healthy lambs, the ileal FAE shows strong carbonic anhydrase reactivity in the apical plasma cell membrane and along the basolateral membrane, even though the basolateral membrane itself is negative (25, 27). Carbonic anhydrase-positive material is also found in vesicles located in the apical cytoplasm of epithelial cells. These findings indicate that carbonic anhydrase-rich particles may contain components of the apical cell membrane (27). The present study demonstrates that infected lambs had reduced carbonic anhydrase reactivity in the FAE 3 weeks after infection, and this result may imply a reduced production of membrane-bound particles. Five of six infected lambs also had reduced granular staining for carbonic anhydrase in the follicle centers. Fusions between the epithelium covering the domes and the adjacent villous epithelium might cause changes in structure and impaired function of the FAE. In addition, degeneration of cells and increased cell turnover in the FAE might result in replacement by immature cells. These factors could have contributed to the reduced carbonic anhydrase staining of FAE found in infected lambs in experiment 2. Thus, reduced production of membrane-bound particles and the consequent decrease in a potential lymphoproliferative stimulus may be of significance for lymphocyte depletion.
Damage to the FAE seems to be a general feature of many infectious diseases (2, 4, 15, 20, 23, 26, 29, 32, 48), and bacterial and viral pathogens may in fact have an apparent predilection for this epithelium. Localization of protozoan parasites to the FAE has been reported for cryptosporidiosis in calves (23, 31), coccidiosis in rabbits (38, 39), and Isospora sp. infection in piglets (49). The effect of FAE lesions on follicle morphology was not included in these studies, except for cryptosporidiosis in calves, where follicles appeared to be normal (23). Lymphocyte depletion of Peyer’s patch follicles has been reported for severe ovine coccidiosis (8), and increased migration of follicle lymphocytes to the intestinal mucosa has been suggested as the cause (11).
The increased reactivity for 5′-nucleotidase observed in the IPP follicles of infected lambs in experiment 2 was probably due to increased loss of lymphocytes and/or decreased proliferation of lymphocytes. This conclusion was supported by immunocytochemical analysis for various leukocyte markers, such as CD45 (leukocyte common antigen), and B-cell markers (CD21 and BLA.36), which all showed decreased numbers of positive cells in depleted follicles. Staining for the cell proliferation antigen Ki-67 confirmed the reduced proliferation of B cells in the IPP follicles of five of six lambs 3 weeks after infection, whereas lambs examined 2 weeks after infection had apparently normal staining for Ki-67. Follicles with severe lymphocyte depletion had moderately increased staining for the macrophage marker CD68, a result that may reflect increased numbers of macrophages in IPP follicles.
Various other factors in addition to FAE lesions may have contributed to the reduced population of follicular B cells in infected lambs 3 weeks after infection. Vigorous B-cell production normally takes place in the IPP in lambs (42, 44), and less than 5% of IPP B cells differentiate and emigrate (41). Apoptosis of follicular lymphocytes is a normal event in the IPP of healthy lambs, and the great majority of ileal B lymphocytes undergo apoptosis (34, 41). Numerous apoptotic cells are present in normal ileal lymphoid follicles and were also present in control lambs and infected lambs in experiment 1 in the present study. In contrast, infected lambs in experiment 2 had decreased numbers of apoptotic cells in the follicles. The present study does not provide direct evidence that apoptotic events play a major role in the lymphocyte depletion of IPP follicles in ovine coccidiosis. If apoptosis were important for lymphocyte depletion in infected lambs, it most likely would take place in an early phase of the disease. Cell death by apoptosis can be quickly induced and was increased in the IPP B-cell population as early as 16 h after a dexamethasone injection in lambs (33). Apoptotic bodies are usually rapidly removed and engulfed by phagocytes. The increased numbers of macrophages in follicles with severe lymphocyte depletion may suggest that apoptosis contributes to cell depletion.
Cell death may also be caused by necrosis, which usually is accompanied by an inflammatory reaction. In the present study, inflammation was not observed in the IPP follicles of infected lambs 3 weeks after infection, except for lamb no. 18, which had intrafollicular abscesses. We therefore believe it unlikely that necrosis of IPP B cells is an important factor in the lymphocyte depletion of follicles. If necrosis were an early event in infection, acute changes would have been expected in the IPP follicles of infected lambs in experiment 1.
In theory, increased emigration of B cells to the intestinal mucosa could result in lymphocyte depletion of IPP follicles. In sheep, emigration of IPP B cells to other sites occurs only to a small extent, and these cells travel to all lymphoid tissues (43). Our experiment did not show a distinct increase in the B-cell population of the intestinal lamina propria or the mesenteric lymph nodes (data not shown). On the basis of the present findings, we suggest that lesions of the FAE may represent a decrease in a lymphopoietic stimulus that may be of major importance for the lymphocyte depletion observed in E. ovinoidalis infection.
Acknowledgments
We thank Inger Rudshaug, Laila Aune, and Ingjerd Andersen for skillful technical assistance. We also thank W. Hein, Wallaceville Animal Research Center, Upper Hutt, New Zealand, for providing monoclonal antibodies used in this study.
REFERENCES
- 1.Aleksandersen, M., T. Landsverk, B. Gjerde, and O. Helle. 1995. Scarcity of gamma delta T cells in intestinal epithelia containing coccidia despite general increase of epithelial lymphocytes. Vet. Pathol. 32:504–512. [DOI] [PubMed] [Google Scholar]
- 2.Autenrieth, I. B., and R. Firsching. 1996. Penetration of M cells and destruction of Peyer’s patches by Yersinia enterocolitica: an ultrastructural and histological study. J. Med. Microbiol. 44:285–294. [DOI] [PubMed] [Google Scholar]
- 3.Bhogal, B. S., E. B. Jacobson, H. Y. Tse, D. M. Schmatz, and O. J. Ravino. 1989. Parasite exposure elicits a preferential T-cell response involved in protective immunity against Eimeria species in chickens primed by an internal-image anti-idiotypic antibody. Infect. Immun. 57:2804–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghesi, C., M. Regoli, E. Bertelli, and C. Nicoletti. 1996. Modifications of the follicle-associated epithelium by short-term exposure to a non-intestinal bacterium. J. Pathol. 180:326–332. [DOI] [PubMed] [Google Scholar]
- 5.Clark, M. A., M. A. Jepson, N. L. Simmons, and B. H. Hirst. 1994. Preferential interaction of Salmonella typhimurium with mouse Peyer’s patch M cells. Res. Microbiol. 145:543–552. [DOI] [PubMed] [Google Scholar]
- 6.Gavrieli, Y., Y. Sherman, and S. A. Ben-Sasson. 1992. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerdes, J., H. Lemke, H. Baisch, H. H. Wacker, U. Schwab, and H. Stein. 1984. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 133:1710–1715. [PubMed] [Google Scholar]
- 8.Gregory, M. W. 1990. Pathology of coccidial infections, p.235–261. In P. L. Long (ed.), Coccidiosis of man and domestic animals. CRC Press, Boca Raton, Fla.
- 9.Gregory, M. W., and J. Catchipole. 1990. Ovine coccidiosis: the pathology of Eimeria crandallis infection. Int. J. Parasitol. 20:849–860. [DOI] [PubMed] [Google Scholar]
- 10.Gregory, M. W., and J. Catchpole. 1986. Coccidiosis in rabbits: the pathology of Eimeria flavescens infection. Int. J. Parasitol. 16:131–145. [DOI] [PubMed] [Google Scholar]
- 11.Gregory, M. W., and J. Catchpole. 1987. Ovine coccidiosis: pathology of Eimeria ovinoidalis infection. Int. J. Parasitol. 17:1099–1111. [DOI] [PubMed] [Google Scholar]
- 12.Griebel, P. J., and W. R. Hein. 1996. Expanding the role of Peyer’s patches in B-cell ontogeny. Immunol. Today 17:30–39. [DOI] [PubMed] [Google Scholar]
- 13.Hein, W. R., L. Dudler, and C. R. Mackay. 1989. Surface expression of differentiation antigens on lymphocytes in the ileal and jejunal Peyer’s patches of lambs. Immunology 68:365–370. [PMC free article] [PubMed] [Google Scholar]
- 14.Hein, W. R., L. Dudler, W. L. Marston, T. Landsverk, A. J. Young, and D. Avila. 1998. Ubiquitination and dimerization of complement receptor type 2 on sheep B cells. J. Immunol. 161:458–466. [PubMed] [Google Scholar]
- 15.Jensen, V. B., J. T. Harty, and B. D. Jones. 1998. Interactions of the invasive pathogens Salmonella typhimurium, Listeria monocytogenes, and Shigella flexneri with M cells and murine Peyer’s patches. Infect. Immun. 66:3758–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, B. D., N. Ghori, and S. Falkow. 1994. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J. Exp. Med. 180:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelley, L. C., E. A. Mahaffey, D. I. Bounous, D. F. Antezak, and R. L. Brooks, Jr. 1997. Detection of equine and bovine T- and B-lymphocytes in formalin-fixed paraffin-embedded tissues. Vet. Immunol. Immunopathol. 57:187–200. [DOI] [PubMed] [Google Scholar]
- 18.Kelly, P. M., E. Bliss, J. A. Morton, J. Burns, and J. O. McGee. 1988. Monoclonal antibody EBM/11: high cellular specificity for human macrophages. J. Clin. Pathol. 41:510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krakowka, S., R. J. Higgins, and A. Koestner. 1980. Canine distemper virus: review of structural and functional modulations in lymphoid tissues. Am. J. Vet. Res. 41:284–292. [PubMed] [Google Scholar]
- 20.Landsverk, T. 1981. Peyer’s patches and the follicle-associated epithelium in diarrheic calves. Pathomorphology, morphometry and acid phosphatase histochemistry. Acta Vet. Scand. 22:459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landsverk, T. 1981. The epithelium covering Peyer’s patches in young milk-fed calves. An ultrastructural and enzyme histochemical investigation. Acta Vet. Scand. 22:198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landsverk, T. 1987. The follicle-associated epithelium of the ileal Peyer’s patch in ruminants is distinguished by its shedding of 50 nm particles. Immunol. Cell Biol. 65:251–261. [DOI] [PubMed] [Google Scholar]
- 23.Landsverk, T. 1987. Cryptosporidiosis and the follicle-associated epithelium over the ileal Peyer’s patch in calves. Res. Vet. Sci. 42:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landsverk, T. 1988. Phagocytosis and transcytosis by the follicle-associated epithelium of the ileal Peyer’s patch in calves. Immunol. Cell Biol. 66:261–268. [DOI] [PubMed] [Google Scholar]
- 25.Landsverk, T., A. Jansson, L. Nicander, and L. Ploen. 1987. Carbonic anhydrase—a marker for particles shed from the epithelium to the lymphoid follicles of the ileal Peyer’s patch in goat kids and lambs. Immunol. Cell Biol. 65 Pt 5:425–9:425–429. [DOI] [PubMed] [Google Scholar]
- 26.Landsverk, T., B. Lium, J. A. Matovelo, E. Liven, and K. Nordstoga. 1990. Peyer’s patches in experimental Salmonella dublin infection in calves. Microvascular and epithelial changes contributing to atrophy of lymphoid follicles. APMIS 98:255–268. [PubMed] [Google Scholar]
- 27.Landsverk, T., W. Trevella, and L. Nicander. 1990. Transfer of carbonic anhydrase-positive particles from the follicle-associated epithelium to lymphocytes of Peyer’s patches in foetal sheep and lambs. Cell Tissue Res. 261:239–247. [DOI] [PubMed] [Google Scholar]
- 28.Larsen, H. J., and T. Landsverk. 1986. Distribution of T and B lymphocytes in jejunal and ileocaecal Peyer’s patches of lambs. Res. Vet. Sci. 40:105–111. [PubMed] [Google Scholar]
- 29.Liebler, E. M., C. Kusters, and J. F. Pohlenz. 1995. Experimental mucosal disease in cattle: changes of lymphocyte subpopulations in Peyer’s patches and in lymphoid nodules of large intestine. Vet. Immunol. Immunopathol. 48:233–248. [DOI] [PubMed] [Google Scholar]
- 30.Maddox, J. F., C. R. Mackay, and M. R. Brandon. 1985. The sheep analogue of leucocyte common antigen (LCA). Immunology 55:347–353. [PMC free article] [PubMed] [Google Scholar]
- 31.Marcial, M. A., and J. L. Madara. 1986. Cryptosporidium: cellular localization, structural analysis of absorptive cell-parasite membrane-membrane interactions in guinea pigs, and suggestion of protozoan transport by M cells. Gastroenterology 90:583–594. [DOI] [PubMed] [Google Scholar]
- 32.Momotani, E., D. L. Whipple, A. B. Thiermann, and N. F. Cheville. 1988. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer’s patches in calves. Vet. Pathol. 25:131–137. [DOI] [PubMed] [Google Scholar]
- 33.Motyka, B., H. S. Bhogal, and J. D. Reynolds. 1995. Apoptosis of ileal Peyer’s patch B cells is increased by glucocorticoids or anti-immunoglobulin antibodies. Eur. J. Immunol. 25:1865–1871. [DOI] [PubMed] [Google Scholar]
- 34.Motyka, B., and J. D. Reynolds. 1991. Apoptosis is associated with the extensive B cell death in the sheep ileal Peyer’s patch and the chicken bursa of Fabricius: a possible role in B cell selection. Eur. J. Immunol. 21:1951–1958. [DOI] [PubMed] [Google Scholar]
- 35.Muller-Hermelink, H. K. 1974. Characterization of the B-cell and T-cell regions of human lymphatic tissue through enzyme histochemical demonstration of ATPase and 5′-nucleotidase activities. Virchows Arch. B Cell Pathol. 16:371–378. [DOI] [PubMed] [Google Scholar]
- 36.Mutwiri, G., T. Watts, L. Lew, T. Beskorwayne, Z. Papp, M. E. Baca-Estrada, and P. Griebel. 1999. Ileal and jejunal Peyer’s patches play distinct roles in mucosal immunity of sheep. Immunology 97:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neutra, M. R. 1998. Current concepts in mucosal immunity. V. Role of M cells in transepithelial transport of antigens and pathogens to the mucosal immune system. Am. J. Physiol. 274:G785–G791. [DOI] [PubMed] [Google Scholar]
- 38.Pakandl, M., P. Coudert, and D. Licois. 1993. Migration of sporozoites and merogony of Eimeria coecicola in gut-associated lymphoid tissue. Parasitol. Res. 79:593–598. [DOI] [PubMed] [Google Scholar]
- 39.Pakandl, M., K. Gaca, F. Drouet-Viard, and P. Coudert. 1996. Eimeria coecicola Cheissin 1947: endogenous development in gut-associated lymphoid tissue. Parasitol. Res. 82:347–351. [DOI] [PubMed] [Google Scholar]
- 40.Reynaud, C. A., C. Garcia, W. R. Hein, and J. C. Weill. 1995. Hypermutation generating the sheep immunoglobulin repertoire is an antigen-independent process. Cell 80:115–125. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds, J. D. 1986. Evidence of extensive lymphocyte death in sheep Peyer’s patches. I. A comparison of lymphocyte production and export. J. Immunol. 136:2005–2010. [PubMed] [Google Scholar]
- 42.Reynolds, J. D. 1987. Peyer’s patches and the early development of B lymphocytes. Curr. Top. Microbiol. Immunol. 135:43–56:43–56. [DOI] [PubMed] [Google Scholar]
- 43.Reynolds, J. D., L. Kennedy, J. Peppard, and R. Pabst. 1991. Ileal Peyer’s patch emigrants are predominantly B cells and travel to all lymphoid tissues in sheep. Eur. J. Immunol. 21:283–289. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds, J. D., and B. Morris. 1983. The evolution and involution of Peyer’s patches in fetal and postnatal sheep. Eur. J. Immunol. 13:627–635. [DOI] [PubMed] [Google Scholar]
- 45.Ridderstrale, Y. 1976. Intracellular localization of carbonic anhydrase in the frog nephron. Acta Physiol. Scand. 98:465–469. [DOI] [PubMed] [Google Scholar]
- 46.Rose, M. E. 1987. Eimeria, Isospora, and Cryptosporidium, p. 276–312. In E. J. L. Soulsby (ed.), Immune responses in parasitic infections: immunology, immunopathology and immunoprophylaxis. CRC Press, Inc., Boca Raton, Fla.
- 47.Sansonetti, P. J., G. Tran Van Nhieu, and C. Egile. 1999. Rupture of the intestinal epithelial barrier and mucosal invasion by Shigella flexneri. Clin. Infect. Dis. 28:466–475. [DOI] [PubMed] [Google Scholar]
- 48.Torres-Medina, A. 1984. Effect of rotavirus and/or Escherichia coli infection on the aggregated lymphoid follicles in the small intestine of neonatal gnotobiotic calves. Am. J. Vet. Res. 45:652–660. [PubMed] [Google Scholar]
- 49.Vitovec, J., and B. Koudela. 1987. Pathology of natural isosporosis in nursing piglets. Folia Parasitol. (Prague) 34:199–204. [PubMed] [Google Scholar]
- 50.Wakelin, D., and M. E. Rose. 1990. Immunity to coccidiosis, p. 282–306. In P. L. Long (ed.), Coccidiosis of man and domestic animals. CRC Press, Boca Raton, Fla.
- 51.Wassef, J. S., D. F. Keren, and J. L. Mailloux. 1989. Role of M cells in initial antigen uptake and in ulcer formation in the rabbit intestinal loop model of shigellosis. Infect. Immun. 57:858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilhelmsen, C. L., S. R. Bolin, J. F. Ridpath, N. F. Cheville, and J. P. Kluge. 1991. Lesions and localization of viral antigen in tissues of cattle with experimentally induced or naturally acquired mucosal disease, or with naturally acquired chronic bovine viral diarrhea. Am. J. Vet. Res. 52:269–275. [PubMed] [Google Scholar]
- 53.Wohlsein, P., G. Trautwein, T. C. Harder, B. Liess, and T. Barrett. 1993. Viral antigen distribution in organs of cattle experimentally infected with rinderpest virus. Vet. Pathol. 30:544–554. [DOI] [PubMed] [Google Scholar]