Abstract
Objective
The ratio of hemoglobin to red blood cell distribution width (Hb/RDW) is a simple and readily available tool associated with adverse outcomes in chronic heart failure (HF). However, the association between the Hb/RDW ratio and mortality in patients with acute decompensated HF (ADHF) is unclear. The goal of this study was to investigate the relationship between the Hb/RDW ratio and mortality in patients after ADHF.
Methods
This single-center study included clinical and laboratory data collected at baseline, with patients prospectively followed-up for a median period of 3.1 years. The patients were divided into two groups based on their median Hb/RDW ratio.
Patients
We evaluated 250 consecutive patients hospitalized for ADHF at Shinshu University Hospital between July 2014 and March 2019.
Results
In our study cohort [median age, 76 (66-83) years; 62.8% male], all-cause death was observed in 91 patients (incidence rate: 12.7 per 100 patient-years). A Kaplan-Meier analysis revealed that patients in the lower Hb/RDW ratio group (<0.24, n=131) had worse outcomes compared to those in the higher group (≥0.24, n=119) (cumulative incidence 44.1% vs. 19.5%, respectively; log-rank, p<0.001). After adjusting for demographics, HF severity, and laboratory biomarkers, a lower Hb/RDW ratio was significantly associated with a higher risk of mortality (hazard ratio, 1.89; 95% confidence interval, 1.04-3.45; p=0.038).
Conclusion
A lower Hb/RDW ratio is associated with an increased risk of mortality in patients after ADHF, thus indicating its potential utility in identifying patients at an elevated risk for future cardiovascular events.
Keywords: heart failure, red blood cell distribution width, prognosis
Introduction
Heart failure (HF) is an increasingly prevalent health care challenge in the aging population and it is associated with increased morbidity and mortality. Acute decompensated HF (ADHF) causes millions of hospitalizations each year. Despite therapeutic advancements, the prognosis of patients with ADHF still remains poor (1-3). Anemia is a frequent comorbidity in these patients, and hemoglobin (Hb) has been identified as an independent prognostic factor in HF (4-6). The red blood cell distribution width (RDW) is a simple and readily available predictive tool that reflects variations in erythrocyte size. It has been reported that an elevation in the RDW results from inflammation, oxidative stress, nutritional deficiencies, and an impaired renal function (7-10), and it is recognized to be a prognostic marker for cardiovascular (CV) events. Previous studies have indicated that the ratio of Hb to RDW (Hb/RDW) is a more effective prognostic tool than either variable alone in patients with various chronic illnesses including cancer and ischemic heart disease (11,12). Additionally, in patients with chronic HF, the Hb/RDW ratio has been linked to mortality and HF readmission (13). However, there is a paucity of data regarding the association between the Hb/RDW-standard deviation (SD) and mortality in patients after ADHF. This study aimed to assess the prevalence of a low Hb/RDW-SD ratio and explore its association with mortality in patients with ADHF.
Materials and Methods
Patients and study design
This was a prospective, single-center, and observational study. It was a post-hoc analysis of data from the Clue of Risk Stratification in the Elderly Patients with Heart Failure (CURE-HF) registry, which was a prospective multicenter cohort study conducted in Nagano Prefecture, Japan. The CURE-HF registry contains the data of 1,036 consecutive patients who were hospitalized with a primary diagnosis of ADHF and thereafter discharged after treatment at 15 institutions between July 2014 and March 2019 (14-16). We recruited 251 consecutive patients at Shinshu University Hospital from the CURE-HF registry in this study. We included patients who were admitted for ADHF, demonstrated signs and symptoms of HF, and thus required treatment with intravenous diuretics. Patients aged <20 years and those with acute coronary syndrome were excluded. The study was approved by the Institutional Review Board of the Shinshu University School of Medicine (approval no.: 4237). All participants provided written informed consent prior to enrollment. The study was conducted in accordance with the principles of the Declaration of Helsinki and registered with the University Hospital Medical Information Network (UMIN 000024470).
Data collection and clinical assessment
Following admission, medical treatment was initiated at the discretion of the investigator. Baseline clinical data, including demographics, medical history, laboratory data, and echocardiographic findings, were collected during the compensated state of HF. Follow-up data were acquired from hospital charts and direct contact with patients or their referring physicians. For a thorough assessment of clinical events, additional information was sourced from visits or telephone contact with surviving patients or family members as well as medical records from other hospitals, when necessary, between June 2021 and August 2021. These events were anonymized before the analysis by investigators who were blinded to the participants' identities. Patients were grouped based on the median Hb/RDW-SD value (0.24) into a high-Hb/RDW-SD group and a low-Hb/RDW-SD group.
The primary endpoint of the study was all-cause mortality. CV death was defined as mortality attributable to HF, acute myocardial infarction, cerebral infarction, CV hemorrhage (including non-stroke intracranial hemorrhage or non-traumatic vascular rupture), or sudden cardiac death. Previous hospitalization for HF was determined based on a history of HF diagnosis according to the Framingham criteria (17) and a record of hospitalization for ADHF. Body mass index (BMI) was calculated as the body weight in kilograms divided by the height in meters squared. Hypertension was defined as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or treatment for hypertension. Dyslipidemia was defined as a serum total cholesterol concentration ≥220 mg/dL, low-density lipoprotein cholesterol (LDL-C) level ≥140 mg/dL, or the need for lipid-lowering therapy. Diabetes mellitus was defined as having an HbA1c ≥6.5%, fasting glucose ≥126 mg/dL, random plasma glucose ≥200 mg/dL, or a history of treatment with oral hypoglycemic agents and/or insulin. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation adapted for Japanese patients. Chronic kidney disease (CKD) was defined as eGFR <60 mL/min/1.73 m2. The left ventricular ejection fraction (LVEF) was determined by echocardiography using the modified Simpson's method.
Statistical analysis
Continuous variables are summarized as the mean±standard deviation or as medians with interquartile ranges. Categorical variables are expressed as frequencies and percentages. Clinical data comparisons between the two categories (higher Hb/RDW-SD ratio group vs. lower Hb/RDW-SD ratio group) were conducted using either the unpaired Student's t-test or the Mann-Whitney U test. The clinical factors associated with a lower Hb/RDW-SD ratio were evaluated using backward stepwise regression models with a p value threshold of 0.05. These included variables such as age, sex, BMI, hypertension, dyslipidemia, diabetes mellitus, prior coronary artery disease, prior stroke, peripheral artery disease, atrial fibrillation, chronic obstructive pulmonary disease, smoking status, New York Heart Association (NYHA) functional class, LVEF, log B-type natriuretic peptide (BNP), albumin, and CKD. Relationships between continuous variables were analyzed using Spearman's correlation. To assess the non-linear associations between Hb/RDW ratio and incidence of all-cause death, restricted cubic spline curves with the number of knots necessary to minimize the model Akaike Information Criteria (2-5 knots tested) were used. Kaplan-Meier curves from baseline (date of discharge) to the incidence of all-cause death were generated and compared using the log-rank test. To evaluate the association between the Hb/RDW-SD ratio categories and subsequent clinical events, a multivariable Cox proportional hazards regression analysis was employed in three models: 1) unadjusted; 2) adjusted for clinical risk factors, including age, sex, NYHA functional class at discharge, prior HF hospitalization, ischemic cardiomyopathy, atrial fibrillation, CKD, and LVEF (eight covariates); and 3) further adjusted for log (BNP) (nine variables), providing an estimated hazard ratio (HR) with a 95% confidence interval (CI). We also used a Fine-Gray regression model to adjust for competing risks, with the competing events being non-CV death (for CV death) or CV death (for non-CV death), providing a sub-distribution hazard ratio (SHR) (15,18). Receiver operating characteristics (ROC) curve analysis was used to evaluate the discriminatory capacity of each parameter in predicting all-cause death, which was expressed as the area under the curve (AUC). All statistical analyses were conducted using the SPSS software program version 28.0 (SPSS, Chicago, USA) and STATA software program version 14.1 (StataCorp, College Station, USA).
Results
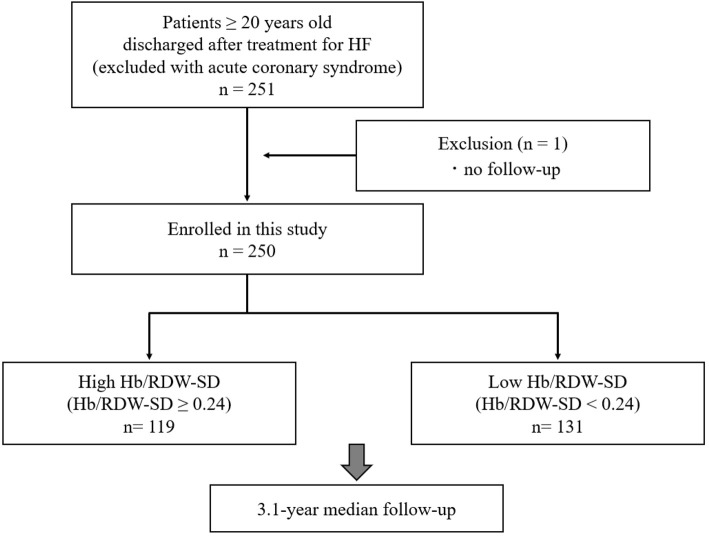
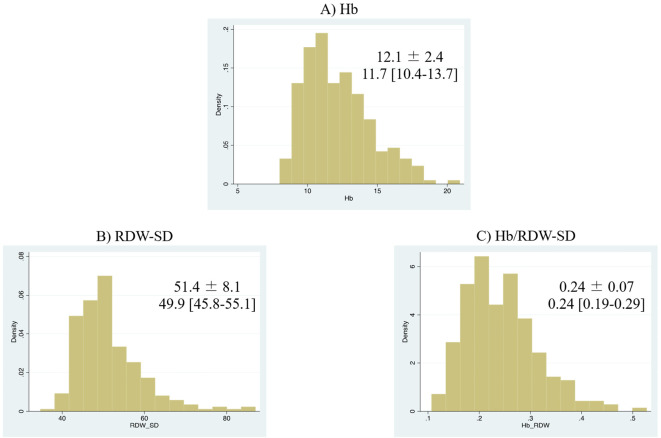
Of the 251 patients after ADHF, one patient with missing follow-up data was excluded from the analysis. Consequently, we assessed a total of 250 patients (99.6%) grouped into the high-Hb/RDW-SD group (n=119) and a low-Hb/RDW-SD group (n=131) (Fig. 1). The baseline clinical characteristics of the patients included in this study are shown in a table (Table 1). The median age of the patients was 76 years, and 62.8% were men. Compared with the 785 CURE-HF patients enrolled in other institutions, our population was younger, was more likely to exhibit a worse NYHA functional class, had a higher history of prior HF and coronary artery disease, and a lower LVEF (Supplemental material 1). The distributions of Hb, RDW-SD, and Hb/RDW-SD are shown (Fig. 2). The median [25th, 75th percentile] and mean±standard deviation values of Hb/RDW-SD were 0.24 [0.19, 0.29] and 0.24±0.07, respectively. On average, patients in the lower Hb/RDW-SD ratio group were older than those in the higher ratio group. Additionally, patients with a lower ratio were more likely to exhibit a worse NYHA functional class and a higher history of prior HF, dementia, and malignant tumors, along with lower levels of albumin, eGFR, and BMI. BNP levels and LVEF values were elevated in patients with lower Hb/RDW-SD ratios. No significant differences were observed in the prevalence of hypertension, dyslipidemia, diabetes mellitus, prior coronary artery disease, atrial fibrillation, or chronic obstructive pulmonary disease between the two groups. The use of medications, including HF medications (angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, beta-blockers, mineralocorticoid receptor antagonists, and sodium-glucose cotransporter 2 inhibitors) and antithrombotic therapy (aspirin, thienopyridines, warfarin, and direct oral anticoagulants) were comparable between the groups. Table 2 presents the determinants of a lower Hb/RDW-SD ratio as assessed using multivariable logistic regression models. The factors significantly associated with a lower Hb/RDW-SD ratio included female sex, CKD, prior stroke, lower albumin levels and BMI, and higher LVEF. During the median 3.1-year follow-up period (interquartile range 1.8-3.8 years), there were 91 all-cause deaths (incidence rate: 12.7 per 100 person-years), 63 CV deaths (incidence rate: 8.8 per 100 person-years), and 28 non-CV deaths (incidence rate: 3.9 per 100 person-years). A lower Hb/RDW ratio was significantly associated with an increased risk of mortality. This association was non-linear, and the relative plateau risk with a lower Hb/RDW ratio was observed at values <0.20-0.22 (Fig. 3). A Kaplan-Meier analysis revealed that patients in the lower Hb/RDW-SD ratio group experienced worse outcomes than those in the higher ratio group (log-rank: p<0.05) (Fig. 4). The lower Hb/RDW-SD ratio group was associated with a higher incidence of CV death (log-rank: p<0.05) and non-CV death (log-rank: p<0.05) (Fig. 5). A lower Hb/RDW ratio was associated with non-CV death, even the competing risk (CV death) was considered (adjusted SHR, 4.11; 95% CI, 1.45-11.62; p=0.008). Conversely, the Hb/RDW ratio was not associated with the risk of CV death when the competing risk (non-CV death) was considered (adjusted SHR, 1.61; 95% CI, 0.93-2.77; p=0.67) (Supplemental material 2).
Figure 1.
Overview of the study design. Clue of Risk Stratification in the Elderly Patients with Heart Failure (CURE-HF) Registry. HF: heart failure, Hb: hemoglobin, RDW-SD: standard deviation of red blood cell distribution width, Hb/RDW-SD: standard deviation of hemoglobin to red blood cell distribution width ratio
Table 1.
Patient Baseline Characteristics according to Hb/RDW-SD Ratio.
| Overall (n=250) | High Hb/RDW-SD (≥0.24) (n=119) | Low Hb/RDW-SD (<0.24) (n=131) | p value | |
|---|---|---|---|---|
| Hb/RDW-SD | 0.24±0.07 0.24 [0.19-0.29] | 0.30±0.05 0.29 [0.26-0.33] | 0.19±0.03 0.19 [0.17-0.21] | - |
| Hb (g/dL) | 12.1±2.4 11.7 [10.4-13.7] | 14.0±2.0 13.7 [12.4-14.9] | 10.5±1.1 10.4 [9.7-11.1] | - |
| RDW-SD (fL) | 51.4±8.1 49.9 [45.8-55.1] | 46.5±4.4 45.8 [43.4-48.7] | 55.9±8.2 53.1 [50.1-59.4] | - |
| Age (years) | 76 [66-83] | 70 [59-80] | 79 [69-86] | <0.05 |
| Age ≥75 years, n (%) | 130 (52.0) | 47 (39.7) | 83 (63.4) | <0.05 |
| Male sex, n (%) | 157 (62.8) | 91 (76.5) | 66 (50.4) | <0.05 |
| BMI (kg/m2) | 21.5±4.2 | 22.7±4.0 | 20.3±4.0 | <0.05 |
| NYHA functional class (III or IV), n (%) | 68 (27.2) | 22 (18.5) | 46 (35.1) | <0.05 |
| Prior heart failure hospitalization, n (%) | 96 (38.4) | 38 (31.9) | 58 (44.3) | 0.05 |
| Etiology: Ischemic cardiomyopathy, n (%) | 80 (32.0) | 34 (38.6) | 46 (35.1) | 0.27 |
| Comorbidities | ||||
| Hypertension, n (%) | 126 (50.4) | 65 (54.6) | 61 (46.6) | 0.20 |
| Dyslipidemia, n (%) | 75 (30.0) | 37 (31.1) | 38 (29.0) | 0.72 |
| Diabetes mellitus, n (%) | 84 (33.6) | 45 (37.8) | 39 (29.8) | 0.18 |
| Atrial fibrillation, n (%) | 133 (45.2) | 60 (50.4) | 73 (55.7) | 0.40 |
| History of coronary artery disease, n (%) | 94 (37.8) | 41 (34.7) | 53 (40.5) | 0.35 |
| History of cerebrovascular disease, n (%) | 40 (16.1) | 14 (11.8) | 26 (20.0) | 0.08 |
| Chronic kidney disease (eGFR<60 mL/min/1.73 m2), n (%) | 191 (76.4) | 81 (68.1) | 110 (84.0) | <0.05 |
| Anemia (Male: <13 g/dL, Female: <12 g/dL), n (%) | 153 (61.2) | 31 (26.1) | 122 (93.1) | <0.05 |
| COPD, n (%) | 22 (8.8) | 7 (5.9) | 15 (11.5) | 0.12 |
| Dementia, n (%) | 9 (3.6) | 1 (0.8) | 8 (6.1) | <0.05 |
| Malignant tumor, n (%) | 27 (10.8) | 5 (4.2) | 22 (16.9) | <0.05 |
| Medications | ||||
| Antithrombotic therapy, n (%) | 213 (85.2) | 110 (84.0) | 103 (86.6) | 0.57 |
| Aspirin, n (%) | 107 (42.8) | 45 (37.8) | 62 (47.3) | 0.13 |
| Warfarin, n (%) | 106 (42.4) | 52 (43.7) | 54 (41.2) | 0.69 |
| DOAC, n (%) | 51 (20.4) | 28 (23.5) | 23 (17.6) | 0.24 |
| ACE-inhibitors/ARBs, n (%) | 203 (81.2) | 102 (85.7) | 101 (77.1) | 0.08 |
| ACE-inhibitors, n (%) | 151 (60.4) | 78 (65.5) | 73 (55.7) | 0.13 |
| ARBs, n (%) | 55 (22.0) | 26 (21.8) | 29 (22.1) | 0.96 |
| Beta-blockers, n (%) | 184 (73.9) | 92 (78.0) | 92 (70.2) | 0.17 |
| MRA, n (%) | 149 (59.6) | 75 (63.0) | 74 (56.5) | 0.29 |
| SGLT-2 inhibitors, n (%) | 11 (4.4) | 9 (7.6) | 2 (1.5) | <0.05 |
| Loop diuretics, n (%) | 205 (82.0) | 94 (79.0) | 111 (84.7) | 0.24 |
| Tolvaptan, n (%) | 90 (36.0) | 35 (29.4) | 55 (42.0) | <0.05 |
| Proton pomp inhibitors, n (%) | 164 (65.6) | 73 (61.3) | 91 (69.5) | 0.18 |
| NSAIDs, n (%) | 9 (3.6) | 2 (1.7) | 7 (5.3) | 0.12 |
| Oral hypoglycemic agents, n (%) | 46 (18.4) | 27 (22.7) | 19 (14.5) | 0.10 |
| Statins, n (%) | 85 (34.0) | 39 (32.8) | 46 (35.1) | 0.70 |
| Laboratory and echocardiography data | ||||
| WBC (/µL) | 5,389±2,052 | 5,599±2,143 | 5,197±1,954 | 0.06 |
| RBC (×106/µL) | 398.6±83.0 | 453.3±76.7 | 348.9±51.1 | <0.05 |
| HCT (%) | 37.1±6.8 | 42.1±5.8 | 32.5±3.7 | <0.05 |
| MCV (fL) | 93.5±6.3 | 92.7±5.2 | 94.2±7.1 | 0.10 |
| MCH (pg) | 30.5±2.5 | 30.8±2.2 | 30.3±2. | 0.09 |
| MCHC (%) | 32.6±1.2 | 33.2±1.0 | 32.2±4.4 | <0.05 |
| Plt (×104/µL) | 19.7±7.4 | 20.1±6.2 | 19.4±8.4 | 0.14 |
| BNP (pg/mL) | 280.5 [158.7-495.7] | 243.5 [138.9-385.6] | 365.4 [177.9-570.6] | <0.05 |
| Albumin (g/dL) | 3.5±0.5 | 3.8±0.4 | 3.3±0.5 | <0.05 |
| Creatinine (mg/dL) | 1.4±0.8 | 1.2±0.5 | 1.5±1.0 | <0.05 |
| eGFR (mL/min/1.73 m2 surface area) | 43.5 [30.0-58.0] | 49.0 [36.0-64.0] | 39.0 [26.0-53.0] | <0.05 |
| Uric acid (mEq/L) | 6.9±1.9 | 6.7±1.9 | 7.0±2.0 | 0.21 |
| Na (mEq/L) | 138.4±3.4 | 138.2±3.2 | 138.6±3.5 | 0.33 |
| K (mEq/L) | 4.3±0.5 | 4.3±0.5 | 4.3±0.6 | 0.80 |
| HbA1c (%) | 6.1±0.8 | 6.4±0.9 | 5.9±0.6 | <0.05 |
| LDL-C (mg/dL) | 93.0±29.3 | 99.8±30.1 | 86.6±27.2 | <0.05 |
| CRP (mg/dL) | 1.00±2.27 | 0.67±0.14 | 1.28±2.68 | <0.05 |
| Erythropoietin (mIU/mL) (n=154) | 29.6±25.5 | 15.7±8.9 | 60.9±109.3 | <0.05 |
| LVEF (%) | 45.0 [31.0-57.9] | 38.0 [28.0-50.6] | 50.0 [35.8-60.8] | <0.05 |
| HFpEF (LVEF≥50%), n (%) | 95 (38.9) | 31 (26.5) | 64 (50.4) | <0.05 |
| HFmrEF (40%≤ LVEF<50%), n (%) | 48 (19.9) | 26 (21.0) | 22 (18.8) | |
| HFrEF (LVEF<40%), n (%) | 99 (41.1) | 35 (28.2) | 64 (54.7) |
Values are presented as number (%), mean±standard deviation, or median [25th, 75th percentiles].
Hb: hemoglobin, RDW-SD: red blood cell distribution width-standard deviation, NYHA: New York Heart Association, eGFR: estimated glomerular filtration rate, COPD: chronic obstructive pulmonary disease, DOAC: direct oral anticoagulant, ACE: angiotensin-converting enzyme, ARB: angiotensin receptor blocker, MRA: mineralocorticoid receptor antagonist, SGLT: sodium-glucose co-transporter, NSAIDs: non-steroidal anti-inflammatory drugs, HCT: hematopoietic cell transplantation, MCV: mean corpuscular volume, MCH: mean hemoglobin volume, MCHC: mean hemoglobin concentration, BNP: B-type natriuretic peptide, HbA1c: hemoglobin A1c, LDL-C: low density lipoprotein cholesterol, LVEF: left ventricular ejection fraction, HFpEF: heart failure with preserved ejection fraction, HFmrEF: heart failure with mid-range ejection fraction, HFrEF: heart failure with reduced ejection fraction
Figure 2.
Distribution of (A) Hb, (B) RDW-SD, and (C) Hb/RDW-SD. Hb: Hemoglobin, RDW-SD: standard deviation of red blood cell distribution width, Hb/RDW-SD: hemoglobin to standard deviation of red blood cell distribution width ratio
Table 2.
Determinants of Low Hb/RDW-SD Assessed by Multivariable Logistic Regression Models (n=197).
| Variables | Adjusted OR (95% CI) | |Z|-score | p value |
|---|---|---|---|
| Albumin (per 1g/dL decrease) | 5.20 (2.38-11.4) | 4.13 | <0.05 |
| Sex (Female) | 2.71 (1.28-5.71) | 2.61 | <0.05 |
| CKD | 3.17 (1.31-7.52) | 2.56 | <0.05 |
| Stroke | 2.78 (1.05-7.35) | 2.05 | <0.05 |
| LVEF (per 1% increase) | 1.02 (1.00-1.04) | 2.04 | <0.05 |
| BMI (per 1 kg/m2 decrease) | 1.10 (1.00-1.20) | 1.97 | <0.05 |
Variables in the multivariate regression models were selected using backward stepwise regression models with a cut-off p value of 0.05. Initial covariates include age, sex, BMI, hypertension, dyslipidemia, diabetes mellitus, prior coronary artery disease, prior stroke, peripheral arterial disease, atrial fibrillation, COPD, smoking status, NYHA functional class at discharge, LVEF, log BNP, albumin, and CKD.
The Z-score was measured in terms of standard deviation from the mean (expressed per 1 SD).
Hb: hemoglobin, RDW-SD: red blood cell distribution width-standard deviation, CKD: chronic kidney disease, NYHA: New York Heart Association, COPD: chronic obstructive pulmonary disease, BNP: B-type natriuretic peptide, LVEF: left ventricular ejection fraction, BMI: body mass index
Figure 3.
Association between the Hb/RDW-SD ratio and the incidence rate of all-cause death using restricted cubic spline curves. The figure shows the incidence rate of all-cause death (events per 100 person-years) on the left y-axis and the Hb/RDW-SD ratio on the x-axis. The solid curves depict the incidences, with 95% confidence intervals of the estimates. Poisson models were used to estimate the incidence rates. Histograms show the population distribution of the Hb/RDW ratio. Hb/RDW-SD: hemoglobin-to-standard deviation of red blood cell distribution width ratio
Figure 4.
Kaplan-Meier curves for all-cause death according to hemoglobin-to-red blood cell distribution width ratio (Hb/RDW-SD).
Figure 5.
Kaplan-Meier curves for cardiovascular death (A) and non-cardiovascular death (B) according to hemoglobin-to-red blood cell distribution width ratio (Hb/RDW-SD).
Table 3 presents both univariate and multivariate adjusted Cox proportional hazards analyses for all-cause deaths, categorized by the presence of a lower Hb/RDW-SD ratio. In the crude model, a lower Hb/RDW-SD ratio was associated with a higher risk of all-cause death (HR, 2.78; 95% CI, 1.77-4.36; p<0.05). Even after multivariable adjustments in Model 1 (eight covariates) and Model 2 (nine covariates), a lower ratio remained significantly associated with an increased risk of all-cause death (Model 1: HR, 1.83; 95% CI, 1.10-3.06; p<0.05; Model 2: HR, 1.89; 95% CI, 1.04-3.45; p<0.05) (Table 3). Based on the Hb/RDW-SD ratio values in our study, the AUC was 0.66. For predicting all-cause death, the AUC of Hb/RDW-SD ratio was not lower than that of Hb (AUC 0.64; comparison, p=0.66) or RDW-SD (AUC 0.63; comparison, p=0.32) alone.
Table 3.
Cox Proportional Hazard Analysis of All-cause Death by the Presence of Low Hb/RDW-SD.
| Presence of low Hb/RDW-SD | Overall (n=250) | High Hb/RDW-SD (n=119) reference | Low Hb/RDW-SD (n=131) HR (95% CI) p value (vs. High-) |
|---|---|---|---|
| All-cause death | 91 events | 27 events | 64 events |
| Event rate (per 100 patients-years) | 12.7 [10.3, 15.6] | 6.9 [4.7, 10.0] | 19.8 [15.5, 25.3] |
| Unadjusted (n=250) | Reference | 2.78 (1.77-4.36) p<0.05 | |
| Adjusted (n=241) by Model 1 | Reference | 1.83 (1.10-3.06)<0.05 | |
| Adjusted (n=197) by Model 2 | Reference | 1.89 (1.04-3.45)<0.05 |
Model 1. Adjusted for age, sex, NYHA functional class, prior heart failure hospitalization, ischemic cardiomyopathy, atrial fibrillation, chronic kidney disease, and LVEF.
Model 2. Adjusted for age, sex, NYHA functional class, prior heart failure hospitalization, ischemic cardiomyopathy, atrial fibrillation, chronic kidney disease, LVEF, and log BNP.
RDW-SD: red blood cell distribution width-standard deviation, NYHA: New York Heart Association, COPD: chronic obstructive pulmonary disease, BNP: B-type natriuretic peptide, LVEF: left ventricular ejection fraction
Discussion
The present study demonstrated that anemia was prevalent in 61% of patients after ADHF, and those with a lower Hb/RDW-SD ratio at discharge were significantly associated with lower albumin levels, a female sex, CKD, prior stroke, and a higher LVEF. We also established that a lower Hb/RDW-SD ratio was linked to an increased risk of all-cause, CV, and non-CV death in patients after ADHF.
We demonstrated that a lower Hb/RDW-SD ratio was associated with female sex, previous stroke, and a higher LVEF. Similarly, in an Israeli chronic HF cohort study, patients with a lower Hb/RDW ratio were predominantly female and had a history of stroke (13), which is consistent with our observations. A retrospective cohort study from Norway involving 25,992 participants reported that a higher RDW was independently associated with an increased incidence of stroke beyond the scope of anemia and traditional atherosclerotic risk factors (19). However, a higher RDW (indicative of a lower Hb/RDW) has been linked to males in the general population (20). The differences in these results could be attributed to variations in age and underlying disease. Indeed, females have been found to have a higher prevalence of HFpEF than males (21). Additionally, patients with HFpEF are known to have CKD, anemia, and a higher RDW (22,23).
Previous studies have explored the relationship between the Hb/RDW ratio and CV outcomes in HF. Rahamim et al. reported that a lower Hb/RDW ratio was associated with an increased risk of mortality and CV hospitalizations in a cohort of 6,888 patients with chronic HF (mean age, 78 years; 52.0% male) (13). Our analysis builds on this by examining the Hb/RDW-SD ratio in patients recently hospitalized for decompensated HF, and investigating its association with mortality. We found that a lower Hb/RDW-SD ratio correlated with a heightened risk of both CV and non-CV mortality. Older patients with HF are more likely to succumb to non-CV causes, particularly those with HFpEF (24). In our study, the lower Hb/RDW group tended to be older and comprised more patients with HFpEF. Infections emerged as the most frequent cause of non-CV death, accounting for 37.5% (9 of 24 patients) of those in the lower Hb/RDW-SD ratio group who died from non-CV causes. In conditions such as sepsis, similar to HF, lower Hb levels are often linked to malnutrition and a weakened immune response. Additionally, RDW has been associated with poor clinical outcomes in patients with sepsis or influenza (25-28). Previous studies have reported the Hb/RDW ratio to be a predictive factor in patients with sepsis (29,30). Nevertheless, further investigation is necessary to determine whether patients with low Hb/RDW-SD are more susceptible to developing severe infections or are more prone to infection.
Anemia is a frequent comorbidity in patients with HF. Hb is a quantitative marker of anemia, and low Hb levels are associated with poor clinical outcomes in patients with HF (4-6). This association contributes to HF severity, thus reflecting underlying chronic inflammation, malnutrition, iron deficiency, bleeding, and chronic kidney disease (31). The RDW is a qualitative anemia marker that is indicative of erythrocyte size variation. A meta-analysis demonstrated that an increased RDW was an independent predictor of CV events (32). In patients with HF, a higher RDW has been reported as a prognostic marker (33-39). Several hypotheses have been proposed to explain the association between an elevated RDW and an increased risk of adverse clinical outcomes (40). Increased RDW may represent considerable deregulation of erythrocyte homeostasis, associated with impaired erythropoiesis and abnormal red cell survival. It involves various metabolic abnormalities, including inflammation, oxidative stress, nutritional deficiencies, and impaired renal function (7-10). The Hb/RDW ratio is a composite marker that combines Hb and RDW, which indicate the quantitative and qualitative aspects of anemia, respectively. This ratio may be a more robust prognostic factor than that of each marker alone. Its utility has been reported in patients with conditions such as esophageal squamous cell carcinoma (11) and has been proven to be an effective prognostic marker in various diseases, including malignancies, infection, and CV disease (13,29,30,41-46). The Hb/RDW ratio combines information from routine blood tests, offering a simple and accessible means of evaluating the prognostic aspects of HF. Particularly in patients after ADHF, the Hb/RDW ratio is not only a significant marker for mortality, but might also serve as a predictive marker for non-CV death. This would allow clinicians to investigate the potential causes of anemia, including iron deficiency, malnutrition, and inflammation, even in cases where anemia has not yet manifested. Identifying patients at a heightened risk of future CV events and those who might benefit from early intervention becomes more feasible with this measure.
This study is associated with some limitations. First, this was a single-center, observational study. Second, detailed data regarding the causes of anemia were not available. Third, the Hb/RDW-SD ratio was assessed only at the time of discharge without subsequent evaluation of changes during the follow-up period. Fourth, as a post-hoc analysis, the possibility of unmeasured confounding factors influencing the results could not be excluded despite multivariable adjustments. Finally, the relatively small sample size prevented an analysis of whether our exploratory results are applicable across different HF subtypes (HF with reduced ejection fraction versus preserved ejection fraction) and more relevant for predicting non-CV death rather than CV death.
In conclusion, a lower Hb/RDW-SD ratio is associated with an increased risk of mortality in patients after ADHF. These exploratory findings highlight the potential utility of the Hb/RDW-SD ratio in identifying patients at an elevated risk for future CV events.
The authors state that they have no Conflict of Interest (COI).
Supplementary Material
Values are presented as number (%), mean ± standard deviation, or median [25th, 75th percentiles].
Multivariable analyses were adjusted for age and sex.
References
- 1.Yaku H, Ozasa N, Morimoto T, et al.; the KCHF Study Investigators . Demographics, management, and in-hospital outcome of hospitalized acute heart failure syndrome patients in contemporary real clinical practice in Japan - observations from the prospective, multicenter Kyoto Congestive Heart Failure (KCHF) registry -. Circ J 82: 2811-2819, 2018. [DOI] [PubMed] [Google Scholar]
- 2.Shiraishi Y, Kohsaka S, Sato N, et al. 9-year trend in the management of acute heart failure in Japan: a report from the National Consortium of Acute Heart Failure Registries. J Am Heart Assoc 7: e008687, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ide T, Kaku H, Matsushima S, et al.; the JROADHF Investigators . Clinical characteristics and outcomes of hospitalized patients with heart failure from the Large-Scale Japanese Registry Of Acute Decompensated Heart Failure (JROADHF). Circ J 85: 1438-1450, 2021. [DOI] [PubMed] [Google Scholar]
- 4.Horwich TB, Fonarow GC, Hamilton HA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol 39: 1780-1786, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Kajimoto K, Sato N, Takano T. Association between anemia, clinical features and outcome in patients hospitalized for acute heart failure syndromes. Eur Heart J Acute Cardiovasc Care 4: 568-576, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi T, Sakata Y, Takeda T, et al. Prognostic impact of anemia in patients with chronic heart failure - with special reference to clinical background: report from the CHART-2 study -. Circ J 79: 1984-1993, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Lippi G, Turcato G, Cervellin G, Sanchis-Gomar F. Red blood cell distribution width in heart failure: a narrative review. World J Cardiol 10: 6-14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pernow J, Mahdi A, Yang J, Zhou Z. Red blood cell dysfunction: a new player in cardiovascular disease. Cardiovasc Res 115: 1596-1605, 2019. [DOI] [PubMed] [Google Scholar]
- 9.Cole J, Ertoy D, Lin H, et al. Lack of angiotensin II-facilitated erythropoiesis causes anemia in angiotensin-converting enzyme-deficient mice. J Clin Invest 106: 1391-1398, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xanthopoulos A, Giamouzis G, Dimos A, et al. Red blood cell distribution width in heart failure: pathophysiology, prognostic role, controversies and dilemmas. J Clin Med 11: 1951, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun P, Zhang P, Chen C, et al. The ratio of hemoglobin to red cell distribution width as a novel prognostic parameter in esophageal squamous cell carcinoma: a retrospective study from southern China. Oncotarget 7: 42650-42660, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu J, Zhou T, Xue M, et al. Correlation analysis of hemoglobin-to-red blood cell distribution width ratio and frailty in elderly patients with coronary heart disease. Front Cardiovasc Med 8: 728800, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahamim E, Zwas DR, Keren A, et al. The ratio of hemoglobin to red cell distribution width: a strong predictor of clinical outcome in patients with heart failure. J Clin Med 11: 886, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanai M, Minamisawa M, Motoki H, et al. Prognostic impact of hyperpolypharmacy due to noncardiovascular medications in patients after acute decompensated heart failure - insight from the Clue of Risk Stratification in the Elderly Patients With Heart Failure (CURE-HF) registry -. Circ J 88: 33-42, 2024. [DOI] [PubMed] [Google Scholar]
- 15.Machida K, Minamisawa M, Motoki H, et al. clinical profile and prognostic of dementia in patients with acute decompensated heart failure - from the CURE-HF registry -. Circ J 88: 93-102, 2024. [DOI] [PubMed] [Google Scholar]
- 16.Sakai T, Motoki H, Fuchida A, et al. Comparison of prognostic impact of anticoagulants in heart failure patients with atrial fibrillation and renal dysfunction: direct oral anticoagulants versus vitamin K antagonists. Heart Vessels 37: 1232-1241, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKee Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 285: 1441-1446, 1971. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risk. Circulation 133: 601-609, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lappegård J, Ellingsen TS, Skjelbakken T, et al. Red cell distribution width is associated with future risk of incident stroke. The Tromsø Study. Thromb Haemost 115: 126-34, 2016. [DOI] [PubMed] [Google Scholar]
- 20.Zalawadiya SK, Veeranna V, Panaich SS, Afonso L, Ghali JK. Gender and ethnic differences in red cell distribution width and its association with mortality among low risk healthy United State adults. Am J Cardiol 1096: 1664-1670, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Sakata Y, Miyata S, Nochioka K, et al. Gender differences in clinical characteristics, treatment and long-term outcomes in patients with stage C/D heart failure in Japan. Report from the CHART-2 study. Circ J 78: 428-435, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Seni M, Paulus WJ, Gavazzi A, et al. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J 35: 2797-2815, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai R, Uemura Y, Okumura T, et al. Impact of red blood cell distribution width on non-cardiac mortality in patients with acute decompensated heart failure with preserved ejection fraction. J Cardiol 70: 591-597, 2017. [DOI] [PubMed] [Google Scholar]
- 24.Nakamaru R, Shiraishi Y, Sandhu AT, et al. Cardiovascular vs. non-cardiovascular deaths after heart failure hospitalization in young, older, and very old patients. ESC Heart Fail 10: 673-684, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang AY, Ma HP, Kao WF, Tsai SH, Chang CK. Red blood cell distribution width is associated with mortality in elderly patients with sepsis. Am J Emerg Med 36: 949-953, 2018. [DOI] [PubMed] [Google Scholar]
- 26.Moreno-Torres V, Royuela A, Múñez-Rubio E, et al. Red blood cell distribution width as prognostic factor in sepsis: a new use for a classical parameter. J Crit Care 71: 154069, 2022. [DOI] [PubMed] [Google Scholar]
- 27.Sadaka F, O'Brien J, Prakash S. Red cell distribution width and outcome in patients with septic shock. J Intensive Care Med 28: 307-313, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Topaz G, Kitay-Cohen Y, Peled L, et al. The association between red cell distribution width and poor outcomes in hospitalized patients with influenza. J Crit Care 41: 166-169, 2017. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Chen Z, Yang H, Li H, Chen R, Yu J. Relationship between the hemoglobin-to-red cell distribution width ratio and all-cause mortality in septic patients with atrial fibrillation: based on propensity score matching method. J Cardiovasc Dev Dis 9: 400, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang X, Yuan S, Ling Y, et al. The hemoglobin-to-red cell distribution width ratio to predict all-cause mortality in patients with sepsis-associated encephalopathy in the MIMIC-IV database. Int J Clin Pract 7141216, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation 138: 80-98, 2018. [DOI] [PubMed] [Google Scholar]
- 32.Hou H, Sun T, Li C, et al. An overall and dose-response meta-analysis of red blood cell distribution width and CVD outcomes. Sci Rep 7: 43420, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Najjar Y, Goode KM, Zhang J, Cleland JG, Clark AL. Red cell distribution width: an inexpensive and powerful prognostic marker in heart failure. Eur J Heart Fail 11: 1155-1162, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Felker GM, Allen LA, Pocock SJ, et al.; the CHARM Investigators . Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM program and the Duke Databank. J Am Coll Cardiol 50: 40-47, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Jung C, Fujita B, Lauten A, et al. Red blood cell distribution width as useful tool to predict long-term mortality in patients with chronic heart failure. Int J Cardiol 152: 417-418, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Jackson CE, Dalzell JR, Bezlyak V, et al. Red cell distribution width has incremental prognostic value to B-type natriuretic peptide in acute heart failure. Eur J Heart Fail 11: 1152-1154, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Allen LA, Felker GM, Mehra MR, et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail 16: 230-238, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pascual-Figal DA, Bonaque JC, Redondo B, et al. Red blood cell distribution width predicts long-term outcome regardless of anaemia status in acute heart failure patients. Eur J Hear Fail 11: 840-846, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Shao Q, Li L, Li G, Liu T. Prognostic value of red blood cell distribution width in heart failure patients: a meta-analysis. Int J Cardiol 179: 495-499, 2015. [DOI] [PubMed] [Google Scholar]
- 40.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 5: 86-105, 2015. [DOI] [PubMed] [Google Scholar]
- 41.Petrella F, Casiraghi M, Radice D, et al. Prognostic value of the hemoglobin/red cell distribution width ratio in resected lung adenocarcinoma. Cancers 13: 710, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su YC, Wen SC, Li CC, et al. Low hemoglobin-to-red cell distribution width ratio is associated with disease progression and poor prognosis in upper tract urothelial carcinoma. Biomedicines 9: 672, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu F, Yang S, Tang X, Liu W, Chen H, Gao H. Prognostic value of baseline hemoglobin-to-red blood cell distribution width ratio in small cell lung cancer: a retrospective analysis. Thorac Cancer 11: 888-897, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yılmaz H, Yılmaz A, Demirağ G. Prognostic significance of hemoglobin-to-red cell distribution width ratio in patients with metastatic renal cancer. Future Oncol 17: 3853-3864, 2021. [DOI] [PubMed] [Google Scholar]
- 45.Zhai Z, Gao J, Zhu Z, et al. The ratio of the hemoglobin to red cell distribution width combined with the ratio of platelets to lymphocytes can predict the survival of patients with gastric cancer liver metastasis. Biomed Res Int 2021: 8729869, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao W, Shi M, Zhang J. Preoperative hemoglobin-to-red cell distribution width ratio as a prognostic factor in pulmonary large cell neuroendocrine carcinoma: a retrospective cohort study. Ann Transl Med 10: 42, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Values are presented as number (%), mean ± standard deviation, or median [25th, 75th percentiles].
Multivariable analyses were adjusted for age and sex.