Abstract
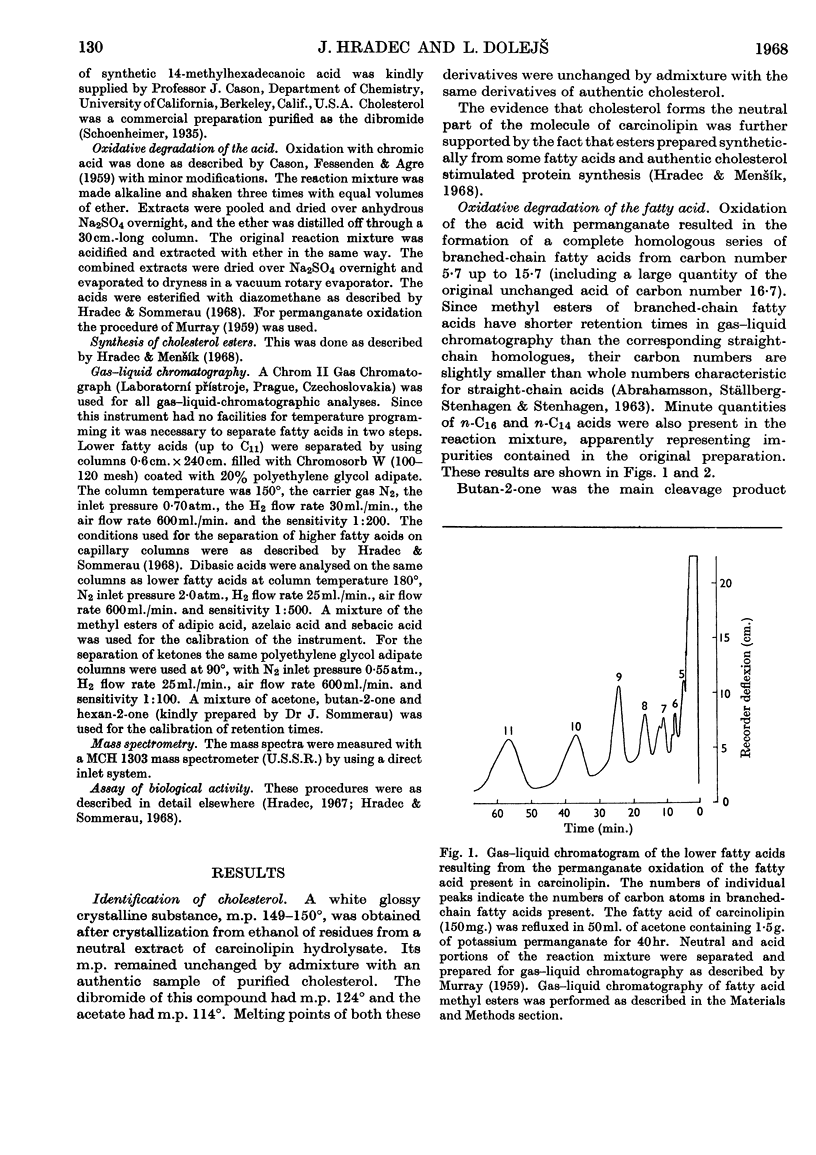
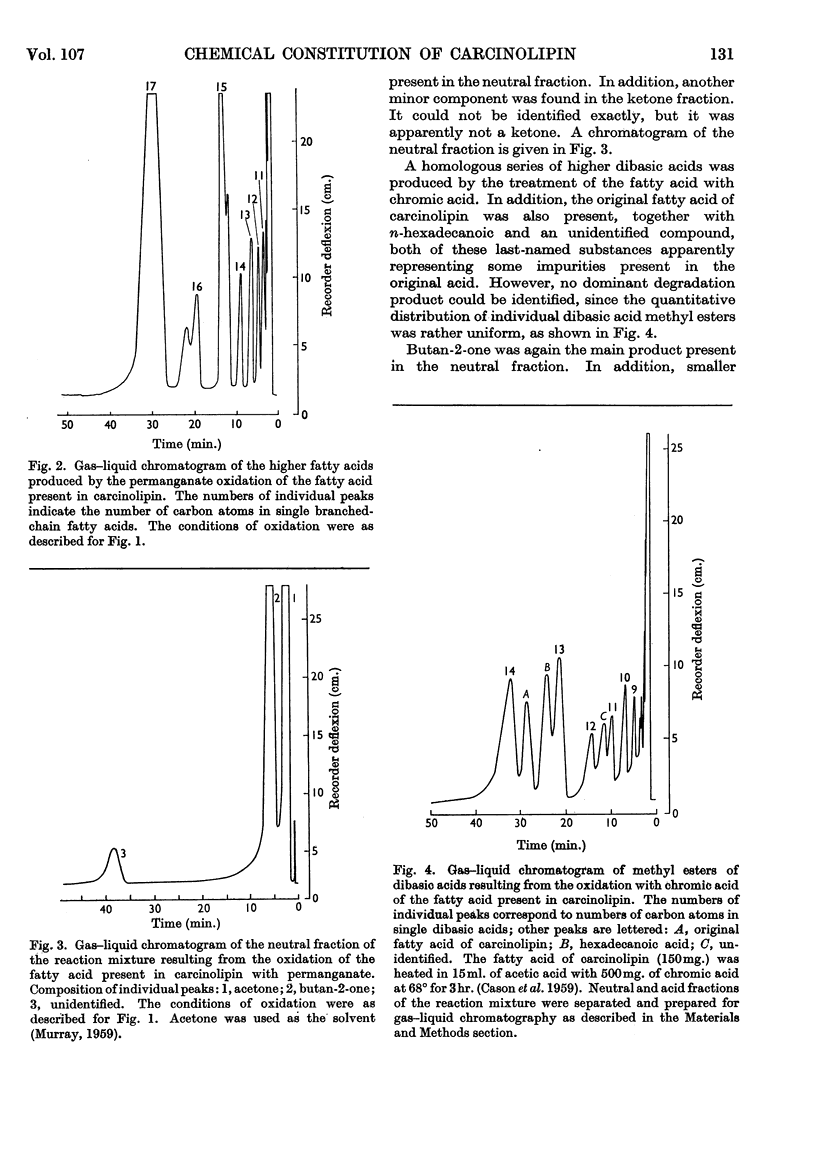
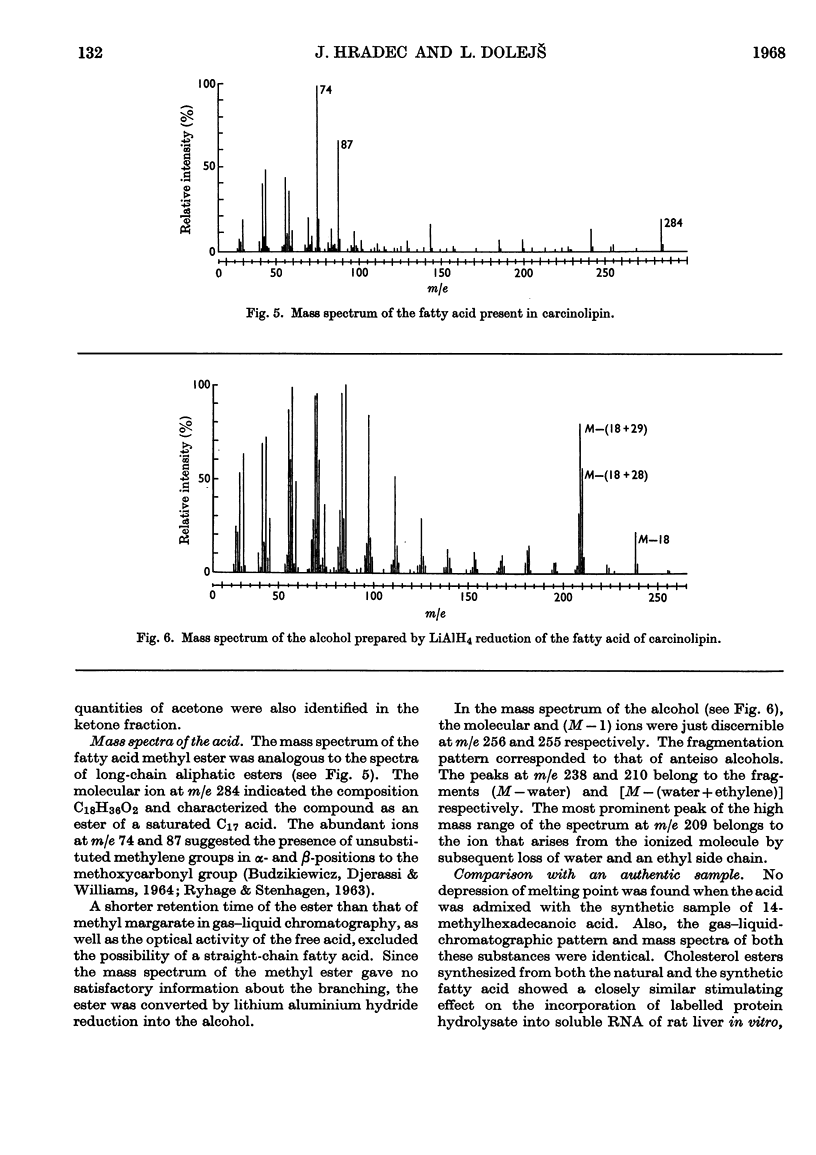
1. The neutral portion of the molecule of carcinolipin was found to be cholesterol by comparison of mixed melting points with cholesterol, its dibromide and its acetate. 2. The fatty acid present in carcinolipin was subjected to oxidative degradation by chromic acid and permanganate. Butan-2-one was the main neutral degradation product resulting from both these procedures. A mixture of dibasic acids was obtained after the oxidation with chromic acid. Permanganate oxidation yielded a complete homologous series of branched-chain C5–C17 fatty acids. 3. The mass spectrum of the acid was characteristic for a saturated C17 acid. The alcohol prepared by lithium aluminium hydride reduction of the original acid showed a mass spectrum typical for an anteiso compound. 4. Comparison of mixed melting points, gas–liquid-chromatographic behaviour and mass spectra of the fatty acid isolated from carcinolipin with an authentic sample of 14-methylhexadecanoic acid demonstrated the identity of these compounds. Cholesterol esters synthesized from authentic cholesterol and the fatty acid isolated from carcinolipin or synthetic 14-methylhexadecanoic acid showed an identical stimulating effect on the incorporation of labelled algal-protein hydrolysate into rat liver transfer RNA in vitro. 5. Mass spectra, results of oxidative degradations and comparisons with an authentic sample, as well as biological activity of the synthetic cholesterol 14-methylhexadecanoate, provided good evidence that carcinolipin is cholesterol (+)-14-methylhexadecanoate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISCHOFF F. CARCINOGENESIS THROUGH CHOLESTEROL AND DERIVATIVES. Prog Exp Tumor Res. 1963;3:412–444. doi: 10.1159/000385969. [DOI] [PubMed] [Google Scholar]
- GRIMMER G., JACOB J. DAS VORKOMMEN METHYLVERZWEIGTER FETTSAEUREN IM HUMANBLUT. Biochem Z. 1965 Feb 24;341:315–324. [PubMed] [Google Scholar]
- HANSEN R. P., SHORLAND F. B., COOKE N. J. The branched-chain fatty acids of mutton fat. I. The isolation of ( )-14-methyl-hexadecanoic acid. Biochem J. 1952 Oct;52(2):203–207. doi: 10.1042/bj0520203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDLER R. W. Some properties and the possible metabolic significance of amino acid-lipid complexes. Biochim Biophys Acta. 1961 May 13;49:297–307. doi: 10.1016/0006-3002(61)90129-9. [DOI] [PubMed] [Google Scholar]
- HRADEC J. Effect of carcinolipin on protein synthesis in cell-free systems. Biochim Biophys Acta. 1961 Feb 12;47:149–157. doi: 10.1016/0006-3002(61)90840-x. [DOI] [PubMed] [Google Scholar]
- Hradec J. Effect of some polycyclic aromatic hydrocarbons on protein synthesis in vitro. Biochem J. 1967 Oct;105(1):251–259. doi: 10.1042/bj1050251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hradec J., Mensík P. Purification of the fatty acid present in carcinolipin. J Chromatogr. 1968 Feb 6;32(3):502–510. doi: 10.1016/s0021-9673(01)80522-7. [DOI] [PubMed] [Google Scholar]
- Hradec J., Sommerau J. Isolation of carcinolipin by combined liquid-solid and liquid-liquid chromatography. J Chromatogr. 1968 Jan 23;32(2):230–242. doi: 10.1016/s0021-9673(01)80489-1. [DOI] [PubMed] [Google Scholar]
- Lederer E. The origin and function of some methyl groups in branched-chain fatty acids, plant sterols and quinones. Biochem J. 1964 Dec;93(3):449–468. doi: 10.1042/bj0930449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serdarevich B., Carroll K. K. Synthesis and characterization of 1- and 2-monoglycerides of anteiso fatty acids. J Lipid Res. 1966 Mar;7(2):277–284. [PubMed] [Google Scholar]



