Abstract
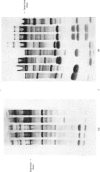
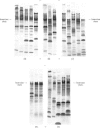
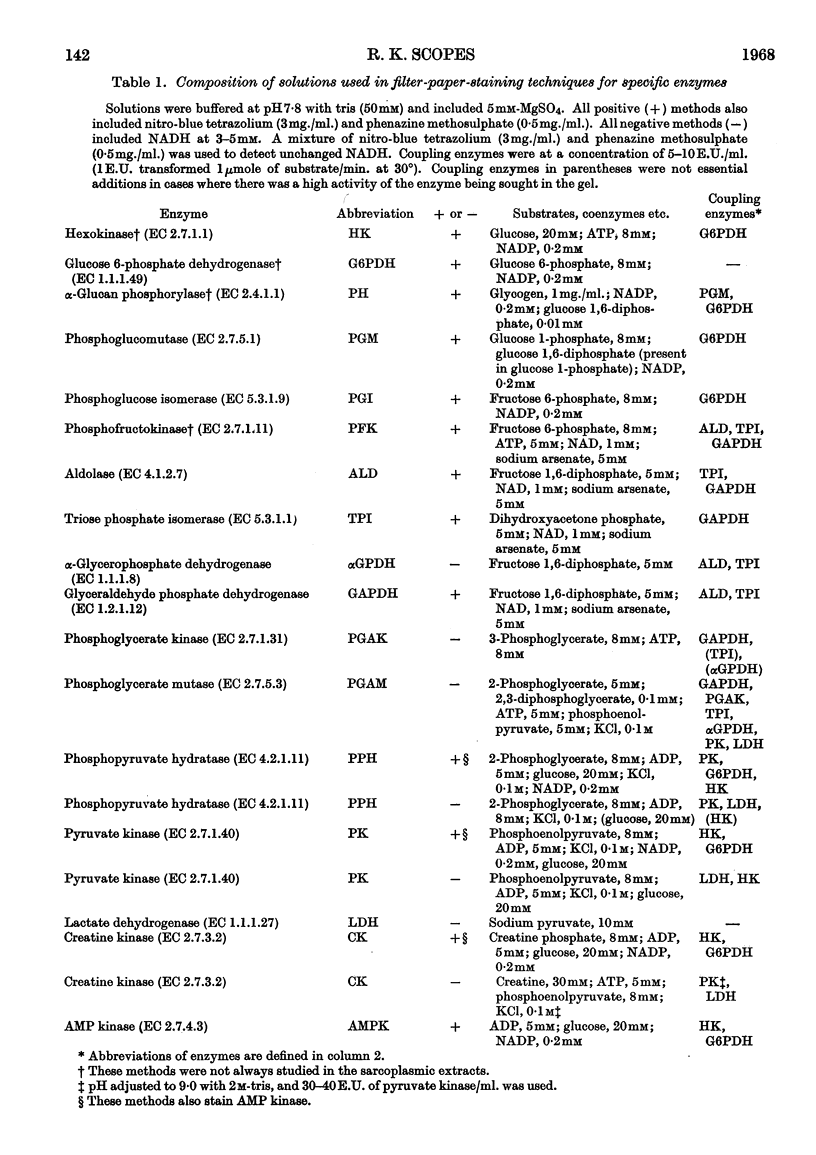
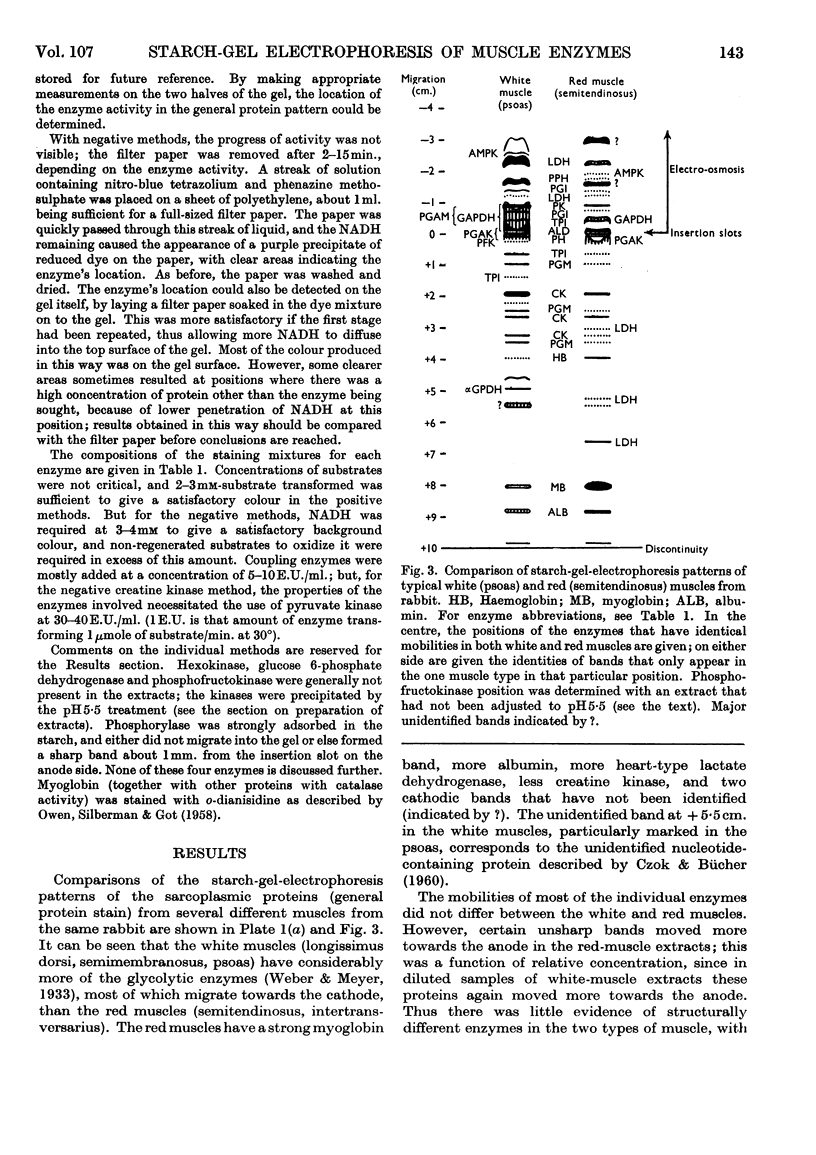
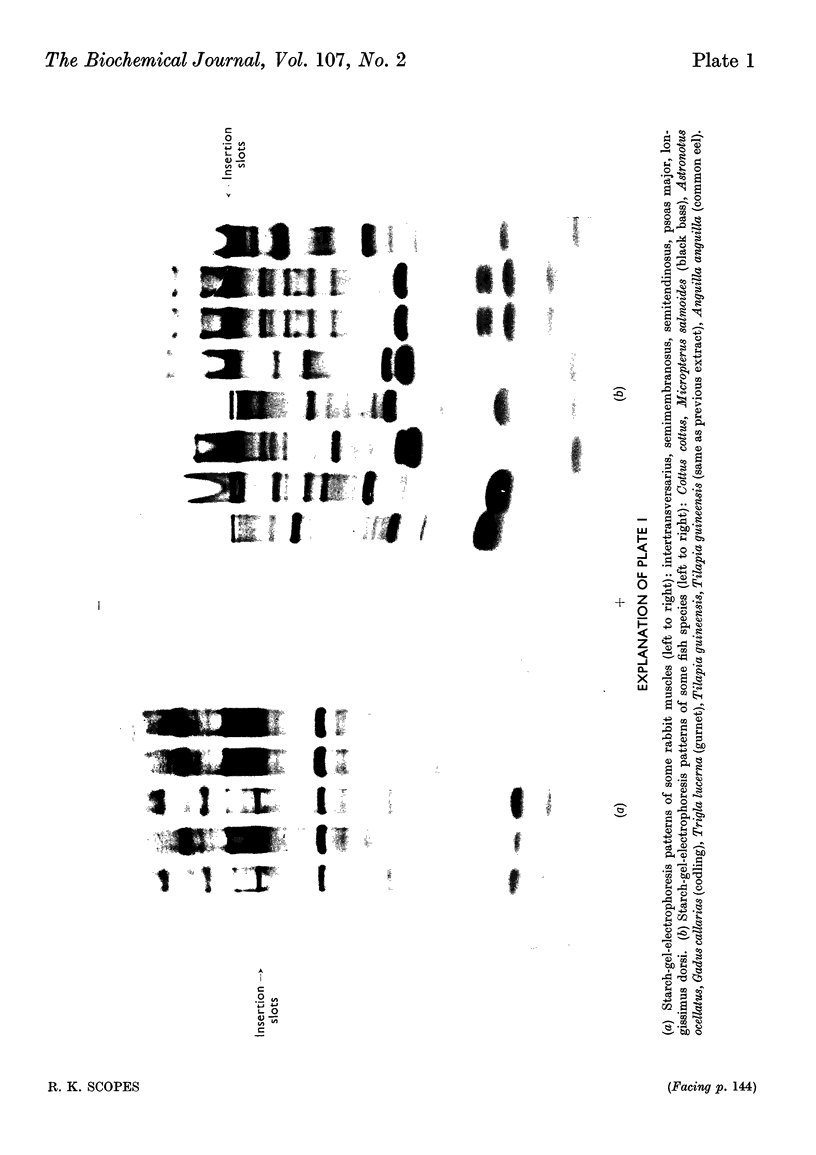
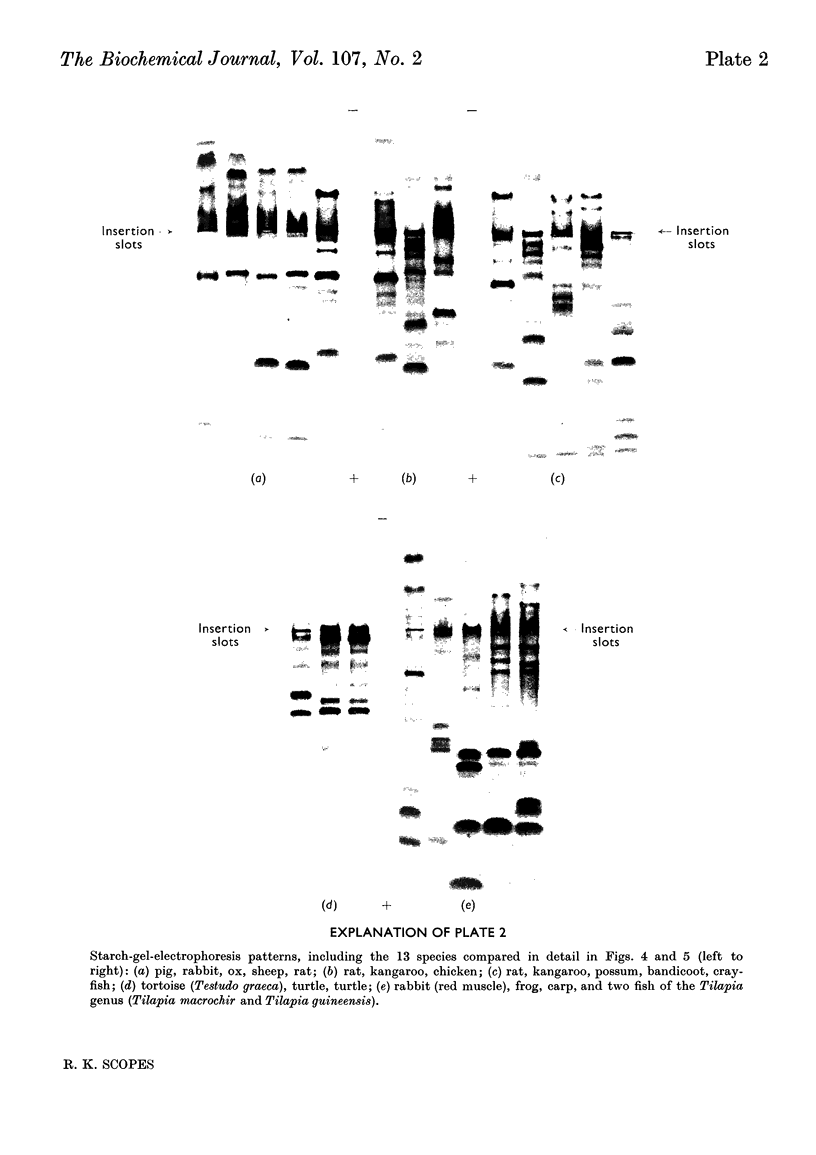
1. Details of an improved method for starch-gel electrophoresis of water-soluble muscle proteins are given. 2. Methods are described for detecting enzyme activities on the starch gel after electrophoresis, by using pieces of filter paper. 3. Compositions of incubation mixtures suitable for detecting any of the enzymes of glycolysis, and certain other enzymes, are given. 4. A comparison of the various enzymes in extracts of several muscles from one rabbit was made; most differences are quantitative only. 5. A detailed comparison of the mobilities of various enzymes in extracts of muscles from a wide variety of species was made. Each species was found to have a characteristic pattern of proteins on the starch gel, and the mobilities of individual enzymes varied considerably. 6. Potential uses and extensions of the methods are discussed.
Full text
PDF

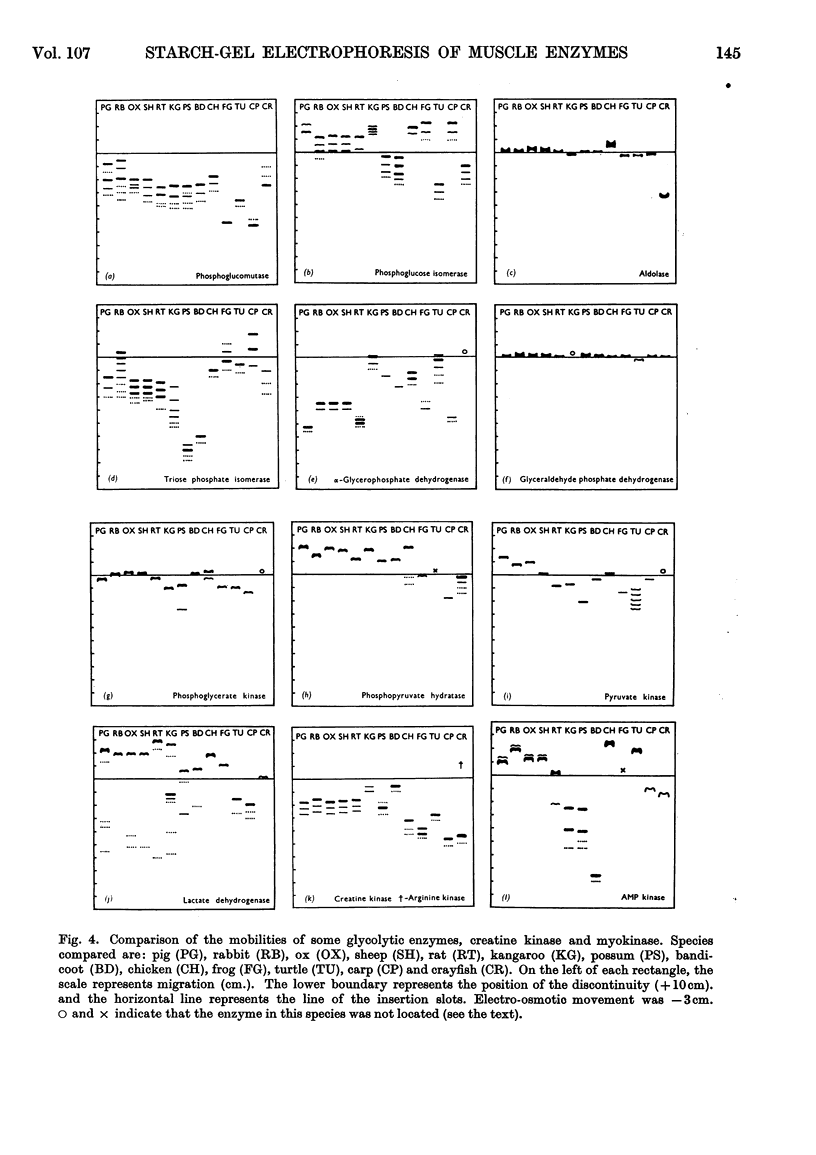


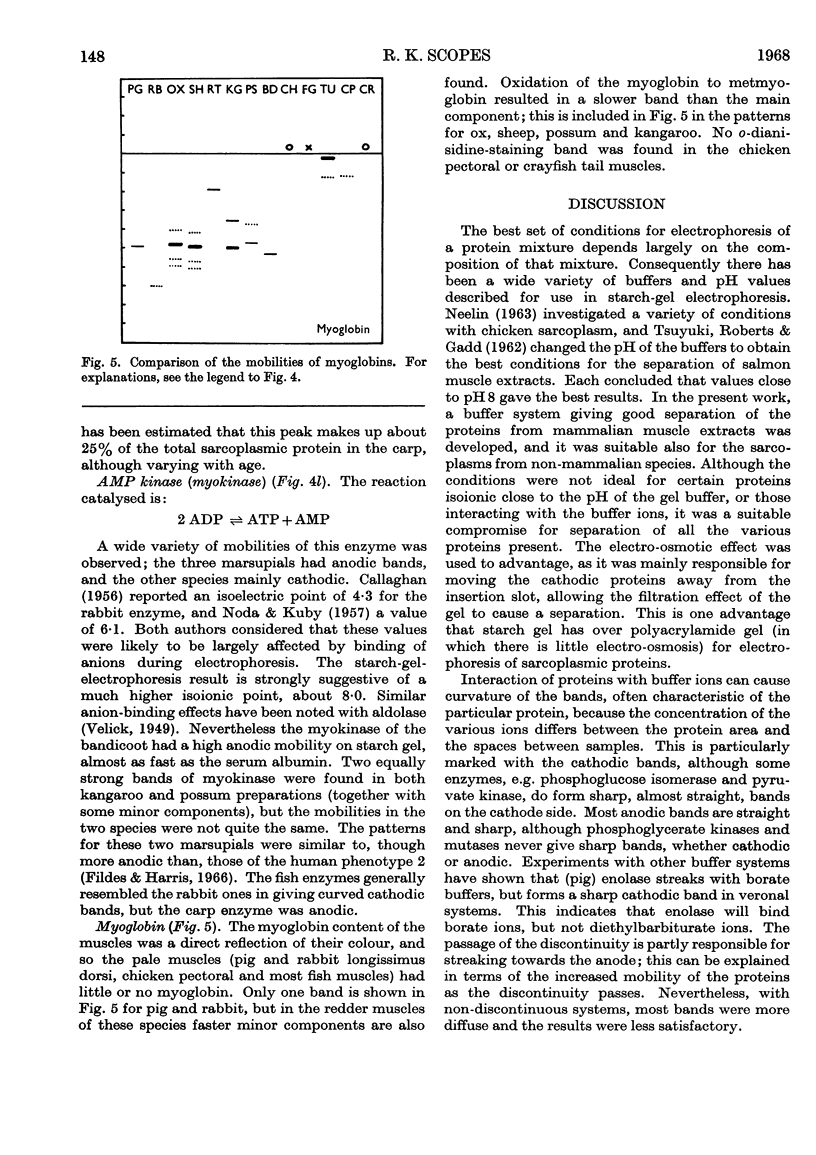


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anstall H. B., Lapp C., Trujillo J. M. Isozymes of aldolase. Science. 1966 Nov 4;154(3749):657–658. doi: 10.1126/science.154.3749.657. [DOI] [PubMed] [Google Scholar]
- Baker C. M. Species, tissue, and individual specificity of low ionic strength extracts of avian muscle and other organs revealed by starch-gel electrophoresis. Can J Biochem. 1966 Jun;44(6):853–859. doi: 10.1139/o66-103. [DOI] [PubMed] [Google Scholar]
- Burton P. M., Waley S. G. The active centre of triose phosphate isomerase. Biochem J. 1966 Sep;100(3):702–710. doi: 10.1042/bj1000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNELL J. J. Studies on the proteins of fish skeletal muscle. II. Electrophoretic analysis of low ionic strength extracts of several species of fish. Biochem J. 1953 Oct;55(3):378–388. doi: 10.1042/bj0550378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREPAX P. Etude électrophorétique d'extraits de muscles doués de différentes propriétés morphologiques et fonctionnelles. Biochim Biophys Acta. 1952 Oct;9(4):385–398. doi: 10.1016/0006-3002(52)90182-0. [DOI] [PubMed] [Google Scholar]
- Eppenberger M. E., Eppenberger H. M., Kaplan N. O. Evolution of creatine kinase. Nature. 1967 Apr 15;214(5085):239–241. doi: 10.1038/214239a0. [DOI] [PubMed] [Google Scholar]
- Fildes R. A., Harris H. Genetically determined variation of adenylate kinase in man. Nature. 1966 Jan 15;209(5020):261–263. doi: 10.1038/209261a0. [DOI] [PubMed] [Google Scholar]
- Focant B., Pechère J. F. Contribution à l'étude des protéines de faible poids moléculaire des myogènes de vertébrés inférieurs. Arch Int Physiol Biochim. 1965 Mar;73(2):334–354. doi: 10.3109/13813456509084256. [DOI] [PubMed] [Google Scholar]
- HAMOIR G. Fish proteins. Adv Protein Chem. 1955;10:227–288. doi: 10.1016/s0065-3233(08)60106-0. [DOI] [PubMed] [Google Scholar]
- HARTSHORNE D. J., PERRY S. V. A chromatographic and electrophoretic study of sarcoplasm from adult--and foetal-rabbit muscles. Biochem J. 1962 Oct;85:171–177. doi: 10.1042/bj0850171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENROTTE J. G. A crystalline constituent from myogen of carp muscles. Nature. 1952 Jun 7;169(4310):968–969. doi: 10.1038/169968b0. [DOI] [PubMed] [Google Scholar]
- HENROTTE J. G. [Contribution to the study of myogens of the carp and the plaice]. Biochim Biophys Acta. 1960 Mar 25;39:103–121. doi: 10.1016/0006-3002(60)90127-x. [DOI] [PubMed] [Google Scholar]
- Jacob J. J. The electrophoretic analysis of protein extracts from striated rabbit muscle. Biochem J. 1947;41(1):83–94. doi: 10.1042/bj0410083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitto G. B., Wilson A. C. Evolution of malate dehydrogenase in birds. Science. 1966 Sep 16;153(3742):1408–1410. doi: 10.1126/science.153.3742.1408. [DOI] [PubMed] [Google Scholar]
- Markert C. L., Møller F. MULTIPLE FORMS OF ENZYMES: TISSUE, ONTOGENETIC, AND SPECIES SPECIFIC PATTERNS. Proc Natl Acad Sci U S A. 1959 May;45(5):753–763. doi: 10.1073/pnas.45.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEELIN J. M. Starch gel electrophoresis of myogen from chicken breast muscle in constant and gradient buffer systems. Can J Biochem Physiol. 1963 Feb;41:369–387. [PubMed] [Google Scholar]
- NIKKILA O. E., LINKO R. R. Paper-electrophoretic analysis of protein extracted at low ionic strength from fish skeletal muscle. Biochem J. 1955 Jun;60(2):242–247. doi: 10.1042/bj0600242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NODA L., KUBY S. A. Adenosine triphosphate-adenosine monophosphate transphosphorylase (myokinase). I. Isolation of the crystalline enzyme from rabbit skeletal muscle. J Biol Chem. 1957 May;226(1):541–549. [PubMed] [Google Scholar]
- Newbold R. P., Scopes R. K. Post-mortem glycolysis in ox skeletal muscle. Effect of temperature on the concentrations of glycolytic intermediates and cofactors. Biochem J. 1967 Oct;105(1):127–136. doi: 10.1042/bj1050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWEN J. A., SILBERMAN H. J., GOT C. Detection of haemoglobin, haemoglobin-haptoglobin complexes and other substances with peroxidase activity after zone electrophoresis. Nature. 1958 Nov 15;182(4646):1373–1373. doi: 10.1038/1821373a0. [DOI] [PubMed] [Google Scholar]
- Odense P. H., Allen T. M., Leung T. C. Multiple forms of lactate dehydrogenase and aspartate aminotransferase in herring (Clupea harengus harengus L.). Can J Biochem. 1966 Oct;44(10):1319–1326. doi: 10.1139/o66-151. [DOI] [PubMed] [Google Scholar]
- POULIK M. D. Starch gel electrophoresis in a discontinous system of buffers. Nature. 1957 Dec 28;180(4600):1477–1479. doi: 10.1038/1801477a0. [DOI] [PubMed] [Google Scholar]
- Parmeggiani A., Luft J. H., Love D. S., Krebs E. G. Crystallization and properties of rabbit skeletal muscle phosphofructokinase. J Biol Chem. 1966 Oct 25;241(20):4625–4637. [PubMed] [Google Scholar]
- Pietruszko R., Baron D. N. A staining procedure for demonstration of multiple forms of aldolase. Biochim Biophys Acta. 1967 Jan 11;132(1):203–206. doi: 10.1016/0005-2744(67)90212-4. [DOI] [PubMed] [Google Scholar]
- ROBERTS E., TSUYUKI H. ZONE ELECTROPHORETIC SEPARATION OF FIVE PHOSPHOGLUCOMUTASE ACTIVITIES FROM FISH MUSCLE. Biochim Biophys Acta. 1963 Aug 6;73:673–676. doi: 10.1016/0006-3002(63)90346-9. [DOI] [PubMed] [Google Scholar]
- SCOPES R. K. ACID DENATURATION OF CREATINE KINASE. Arch Biochem Biophys. 1965 May;110:320–324. doi: 10.1016/0003-9861(65)90126-8. [DOI] [PubMed] [Google Scholar]
- SCOPES R. K. DETECTION OF TRIOSEPHOSPHATE ISOMERASE AFTER ELECTROPHORESIS. Nature. 1964 Feb 29;201:924–925. doi: 10.1038/201924b0. [DOI] [PubMed] [Google Scholar]
- SCOPES R. K. Starch-gel electrophoresis of pig serum proteins. Nature. 1963 Mar 23;197:1201–1201. doi: 10.1038/1971201a0. [DOI] [PubMed] [Google Scholar]
- SJOEVALL K., VOIGT A. CREATINE-PHOSPHO-TRANSFERASE ISOZYMES. Nature. 1964 May 16;202:701–701. doi: 10.1038/202701a0. [DOI] [PubMed] [Google Scholar]
- SPENCER N., HOPKINSON D. A., HARRIS H. PHOSPHOGLUCOMUTASE POLYMORPHISM IN MAN. Nature. 1964 Nov 21;204:742–745. doi: 10.1038/204742a0. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. Isolation and properties of a basic protein from skeletal-muscle sarcoplasm. Biochem J. 1966 Jan;98(1):193–197. doi: 10.1042/bj0980193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopes R. K. The influence of post-mortem conditions on the solubilities of muscle proteins. Biochem J. 1964 Apr;91(1):201–207. doi: 10.1042/bj0910201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. J., GROSSMAN L. I., KAPLAN N. O. Starch-gel electrophoresis of malate dehydrogenase. Biochim Biophys Acta. 1963 Jun 11;73:193–203. doi: 10.1016/0006-3002(63)90303-2. [DOI] [PubMed] [Google Scholar]
- TSUYUKI H. MULTIPLE ELUTION OF PROTEIN ZONES FROM STARCH GEL ELECTROPHORETICALLY. Anal Biochem. 1963 Aug;6:205–209. doi: 10.1016/0003-2697(63)90114-3. [DOI] [PubMed] [Google Scholar]
- TSUYUKI H., ROBERTS E., GADD R. E. Muscle proteins of Pacific salmon (Oncorhynchus). III. The separation of muscle proteins soluble in low ionic strength salt solutions by starch gel electrophoresis. Can J Biochem Physiol. 1962 Jul;40:929–936. [PubMed] [Google Scholar]
- TSUYUKI H., WOLD F. ENOLASE: MULTIPLE MOLECULAR FORMS IN FISH MUSCLE. Science. 1964 Oct 23;146(3643):535–537. doi: 10.1126/science.146.3643.535. [DOI] [PubMed] [Google Scholar]
- Torralba A., Grisolia S. The purification and properties of phosphoglycerate mutase from chicken breast muscle. J Biol Chem. 1966 Apr 25;241(8):1713–1718. [PubMed] [Google Scholar]
- VIRDEN R., WATTS D. C., BALDWIN E. ADENOSINE 5'-TRIPHOSPHATE-ARGININE PHOSPHOTRANSFERASE FROM LOBSTER MUSCLE: PURIFICATION AND PROPERTIES. Biochem J. 1965 Mar;94:536–544. doi: 10.1042/bj0940536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIRDEN R., WATTS D. C. THE DISTRIBUTION OF GUANIDINE-ADENOSINE TRIPHOSPHATE PHOSPHOTRANSFERASES AND ADENOSINE TRIPHOSPHATASE IN ANIMALS FROM SEVERAL PHYLA. Comp Biochem Physiol. 1964 Oct;13:161–177. doi: 10.1016/0010-406x(64)90202-6. [DOI] [PubMed] [Google Scholar]
- Virden R., Watts D. C., Watts R. L., Gammack D. B., Raper J. H. Adenosine 5'-triphosphate-arginine phosphotransferase from lobster muscle. Molecular weight. Biochem J. 1966 Apr;99(1):155–158. doi: 10.1042/bj0990155. [DOI] [PMC free article] [PubMed] [Google Scholar]