Abstract
Fumonisin B1 (FB1), the principal secondary metabolite produced by the fungus Fusarium verticillioides (Gibberella fujikuroi mating population A), is a potent toxin that can be found in fungus-contaminated corn and corn-based food products. We have investigated the immunobiological effects of subchronic dietary exposure to FB1 in male Wistar rats. Animals were fed with diets containing 0 (control) or 100 ppm of FB1 for 12 weeks. The total FB1 intake on day 90 was 810 mg/kg of body weight. Food consumption, body weight, and body weight gain on day 90 were reduced in animals exposed to FB1. Histopathologic changes consisted of histiocytic perivascular infiltrate and an increased number of Kupffer cells in the liver, necrosis and apoptosis of tubular epithelial cells in the kidney, and increased mitotic figures and lymphocytic infiltrate in the small intestine. Serum enzyme alkaline phosphatase was significantly elevated in rats fed FB1, while triglyceride levels decreased compared to controls. Treatment with FB1 in vivo or in vitro did not have a significant effect on mitogen-induced proliferation of spleen mononuclear cells. However, increased levels of interleukin-4 (IL-4) and decreased levels of IL-10 were released by these cells in culture compared to controls. FB1 in vivo or in vitro decreased the hydrogen peroxide (H2O2) released by peritoneal macrophages, while no changes in levels of superoxide anion produced by total peritoneal cells were detected. The results from the present work demonstrate that subchronic FB1 intake could affect the small intestine and alter the interleukin profile and some main functions of macrophages in antitumor activity.
Fumonisins are produced by toxicogenic strains of the genus Fusarium and are synthesized mainly in media where there are nitrogen-limited conditions (37). These mycotoxins have a chemical structure similar to that of ceramides, and it has been shown that they interfere in the lipid metabolism of the cell (30, 40). After isolation and characterization of fumonisin B1 (FB1) and FB2 from cultures of Fusarium verticillioides (F. monoliforme; Gibberella fujikuroi mating population A) strain MRC 826, an interest in these toxins has arisen (3).
Diseases induced by mycotoxins cause acute, chronic, and subchronic toxicities, which depend on different factors such as the animal species, age, sex, strain, dosage, and administration route (18, 41). Fumonisins have been associated with different kinds of mycotoxicoses in domestic animals, such as leukoencephalomalacia in equines (34), pulmonary edema in pigs (10), and hepatocellular carcinoma in rats (15). Animals, as well as humans, are exposed to mycotoxins through consumption of contaminated food in the diet, which can be considered the gateway to cases of natural intoxication by these compounds (17, 19). Contamination with mycotoxins has been detected in different countries in most agricultural products, such as cereals and corn-based food products (16, 25). Of the fumonisins known, only FB1, FB2, and FB3 produce high levels of contamination in naturally contaminated products (16).
During the last few years, different researchers have reported infection levels produced by toxicogenic stocks of Fusarium, Aspergillus, and Penicillium in cereals and in food based on grains produced in Argentina. In these studies Fusarium was found in a high percentage of the analyzed samples. The fumonisin producers F. nygamai (G. fujikuroi mating population G) and F. verticillioides were the main species found (9, 14), with FB1 being the toxin present in the highest concentration (16).
Among the toxins produced by Fusarium, fumonisins, synthesized mainly by F. verticillioides and F. proliferatum (G. fujikuroi mating population D), are the most important because of epidemiological evidence that links them to a high increase of esophageal cancer in humans (33). Marasas et al. have demonstrated a high prevalence of cereals infected by F. verticillioides in African areas where there is a higher incidence of esophageal cancer compared to those with a low incidence of the disease (26).
Dietary exposure to various mycotoxins results in decreases of antibody production, T-lymphocyte proliferative response, cytotoxic action of T lymphocytes, and production of oxygen derivatives by peritoneal cells (8, 31, 44). There is some recent evidence suggesting that FB1 or other structurally related fumonisins are able to modulate the in vivo immune function in broiler chicks. A decrease of viability of lymphocytes in chickens fed an FB1- and FB2-contaminated diet has been reported (12). On the other hand, FB1 and FB2 in vitro are able to induce NO2 production by rat splenic macrophages and to stimulate T-cell proliferation (11).
Other mycotoxins produced by Fusarium, such as vomitoxin (deoxynavaleriol), are able to overinduce interleukin secretion in CD4+ cell cultures, at the same time and in addition to the cell proliferation inhibition (1). Vomitoxin in an experimental macrophage model in vitro also appears to interfere with the associated functions of activated macrophages regulating H2O2 production, depending on the dosage used (20).
Furthermore, a series of microscopic alterations in target organs, such as liver and kidney, has been described. It has been observed that F344 female and male rats that consumed between 0 and 484 ppm of FB1 for 28 days showed apoptosis in liver hyperplasia of the bile ducts and apoptosis of tubular epithelial cells of the kidney (38). According to Bondy et al. (4), the kidney was one of the organs most affected by fumonisin toxicity in male Sprague-Dawley rats, in which they were able to observe necrosis of tubular epithelial cells of the inner cortex, cytoplasmatic basophilia, and atrophy of tubular epithelial cells.
Diets in animals and humans can be contaminated with low levels of fumonisins, producing chronic mycotoxicoses that can alter immunologic mechanisms. The experimental models used to date to study mycotoxin effects on laboratory animals are based mainly on the production of acute mycotoxicoses; however, the alterations at the immunologic level have been studied in only a few cases.
The main objective of this study was to evaluate the immunologic effects caused in rats by an FB1 administration similar to that occurring in nature.
MATERIALS AND METHODS
Animals.
Male Wistar inbred rats, 6 to 8 weeks old, were housed in age-matched pairs in stainless-steel cages. The cages were kept in environmentally controlled rooms with a 12-h light-dark cycle. Animals were housed and cared for in the animal resource facilities of the Department of Clinical Biochemistry, Faculty of Chemical Sciences, National University of Córdoba, in accordance with institutional guidelines.
Preparation of fumonisin extracts.
FB1 was produced using maize as a substratum layer. Wheat (300 g) was placed in 1,000-ml Erlenmeyer flasks at 35% humidity and sterilized for two consecutive days in an autoclave at 121°C for 15 min. A culture of F. verticillioides M 7075 obtained from agar-carnation leaves by monosporic isolation was used as an inoculum. Incubation was for 28 days in the dark at 25°C, with manual stirring during the first 5 days. Separation and purification of the toxin were performed with the fermented wheat, according to a modification of the methodology of Voss et al. (42).
FB1 quantification.
Samples (100 μl) obtained from the extracts were diluted with acetonitrile (100 μl). Before the quantification assays, the samples were diluted 1/50 with acetonitrile-water (1:1). The quantification of the diluted extracts was performed by the methodology proposed by Shephard et al. (36). Briefly, an aliquot (50 μl) of this solution was derivatized with 200 μl of o-phthaldialdehyde. This solution was obtained by adding 5 ml of 0.1 M sodium tetraborate and 50 μl of 2-mercaptoethanol to 1 ml of methanol containing 40 mg of o-phthaldialdehyde. The derivatized samples were analyzed with a high-pressure liquid chromatograph (Hewlett-Packard) equipped with a fluorescence detector. The wavelengths used for excitation and emission were 335 and 440 nm, respectively. An analytical reverse-phase C18 column (150 by 4.6 mm [internal diameter]; 5-μm particle size) connected to a C18 precolumn (20 by 4.6 mm; 5-μm particle size) was used. The mobile phase was methanol-0.1 M NaH2PO4 (75:25), the pH was set at 3.35 ± 0.2 with ortho-phosphoric acid, and a flow rate of 1.5 ml/min was used. The quantification of FB1 was carried out by comparing the peak areas obtained for rats fed FB1 with those corresponding to standards of 10.5, 5.25, and 2.625 μg of FB1 per ml (Programme on Mycotoxins and Experimental Carcinogenesis, Tygerberg, Republic of South Africa).
Diets. (i) Control diet.
The control diet was prepared by adding 435 ml of aqueous extract of maize without inoculation of F. verticillioides to a solution of agar (Difco) (15 g) in 435 ml of distilled water. This mixture was warmed until the agar dilution was completed and then cooled to 50°C. Next, 1,000 g of balanced rat-mouse food (Cargill S.A. C.I., Saladillo, Buenos Aires, Argentina), finely ground and free of mycotoxins, was continuously shaken until a homogeneous mixture was obtained. Pieces of approximately 20 g each were molded, and after solidification they were stored at −18°C until they were used. The final concentration of FB1 in the food was <0.3 ppm.
(ii) Diet with FB1.
The diet with FB1 was prepared as the control diet was, using fumonisin extract as described above. The final FB1 concentration in the food was 100 ppm.
Experimental model.
Two groups of rats were used. One (control) (n = 6) was fed a control diet, and the other (n = 6) was fed a diet with FB1. Animals were housed in pairs in different cages and fed for 90 days. The food ration was replaced daily, and the weights of food portions given and left uneaten after 24 h were determined. Animals were weighed on the 30th, 60th, and 90th days of being fed. After this period, blood samples were obtained by intracardiac puncture and the animals were killed by cervical dislocation.
Determination of food consumption, body weight, body weight gain, and fumonisin consumption.
The food consumption per day was calculated from the difference between the weights of the portions given and uneaten. The body weight was determined on a scale (Ohaus, Florham Park, N.J.) with a precision of 0.05 g. The body weight gain of each animal was determined on the 30th, 60th, and 90th days of feeding as the weight difference in comparison to the weight in the previous month. The total fumonisin consumption on days 30, 60, and 90 for the group given FB1 was calculated by taking into account the food consumption and the toxin concentration in the food. The results are expressed in relation to body weight.
Examination of tissues.
Specimens of lungs, spleen, liver, kidney, and small intestine were obtained on the 90th day of feeding. For examination by light microscopy, tissues were fixed in 10% neutral buffered formalin (pH 7.2). Paraffin sections (4 μm) of tissues were stained with hematoxylin and eosin. Photomicrographs were taken with a Zeiss Axiophot instrument using Kodak Plus-X pan (PX 135 to 24) film. In the small intestine 10 crypts were examined as representatives of each sample, and the numbers of mitotic cells found in the crypt bases of control animals and those fed with FB1 were obtained.
Serum biochemical measurements.
The levels of total cholesterol (Chol), triglycerides (TGs), and calcium (Ca) and the enzymatic activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyltransferase (GGT), and alkaline phosphatase (ALP) in serum obtained from intracardiac puncture were determined by using a Technicon RA-1000 autoanalyzer.
SMCs.
Spleen mononuclear cell (SMC) suspensions were prepared by the method described by Kisaki et al. (22). Briefly, spleens were removed aseptically from animals, minced, and passed through stainless-steel mesh to obtain single-cell suspensions. The cells were washed with RPMI 1640 medium (Sigma) and resuspended in sterile RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (Gibco), gentamicin (50 μg/ml), and β-mercaptoethanol (5 × 10−5 M). Suspensions of spleen cells were prepared aseptically and adjusted to 6 × 106 cells/ml.
Peritoneal cells.
Peritoneal cells were obtained by sterile washing with Krebs Ringer phosphate dextrose buffer (pH 7.0) containing gentamicin (50 mg/liter) and heparin (20 U/ml). Cells were washed twice, resuspended in culture medium, counted, and diluted. Resident cells were collected from rats fed with control diet and from rats fed the FB1 diet.
Mitogenic responses of SMCs.
Cell suspensions (50 μl; 6 × 106 cells/ml; 3 × 105 cells) were dispensed into each well of 96-well culture plates containing 100 μl of culture medium (RPMI 1640). Concanavalin A (ConA) (type IV; Sigma) and lipopolysaccharide (LPS) (055:B5; Sigma) were added at optimal final concentrations of 10 and 40 μg/ml, respectively. The viability of cells was assessed by the trypan blue (0.1%) exclusion test. For in vitro assays, FB1 was added at an optimal final concentration of 10 μM. The cultures were incubated with the mitogens at 37°C in an atmosphere containing 5% CO2 and were labeled during the last 18 h of 96-h cultures with 1 μCi of [3H]thymidine (Comisión Nacional de Energía Atómica). These cells were harvested 18 h thereafter on a glass fiber filter using an automated cell harvester (Skatron; Molecular Devices, Sunnyvale, Calif.). Incorporation of tritiated thymidine into cell DNA was measured in triplicate using a beta liquid scintillation counter.
Cytokine measurement.
SMC suspensions were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (Gibco), gentamicin (50 μg/ml), β-mercaptoethanol (5 × 10−5 M), and ConA (type IV; Sigma), at an optimal final concentration of 10 μg/ml. Supernatants from cultures were collected after 24 h for determination of interleukin-2 (IL-2) and after 72 h for determination of IL-4 and IL-10 and were frozen at −70°C until analyzed. Interleukins were measured using an sandwich enzyme-linked immunosorbent assay protocol (35). Briefly, a purified fraction of anti-IL-2, anti-IL-4, and anti-IL-10 antiserum (PharMingen) was used as capture antibody in conjunction with the biotinylated anti-rat IL-2, IL-4, and IL-10 monoclonal antibody. Dilutions of recombinant rat IL-2, IL-4, and IL-10 were used as standards. After being washed four times with phosphate-buffered saline-Tween 20, the plates were reacted with horseradish peroxidase-streptavidin (Sigma) and o-phenylenediamine was added. After 5 to 20 min, the reaction was stopped with 25 μl of sulfuric acid (1:9, vol/vol). The reactions were read in a microplate reader (Bio-Rad), and results are expressed as nanograms per milliliter.
Detection of H2O2 released by adherent cells.
The phenol red oxidation microassay was used. Briefly, cells (8 × 106/ml) were placed in 96-well plates and left to stand for 2 h at 37°C in 5% CO2. The medium was then replaced with 250 μl of PRS buffer (NaCl [140 mM], dextrose [5.5 mM], phenol red [280 μM], and peroxidase [Sigma] [EC 1.11.1.7] [8.5 U/ml] in phosphate-buffered saline, pH 7.0). For the in vitro assays, FB1 was added at an optimal final concentration of 10 μM (7.21 μg/ml). Wells were treated with phorbol 12-myristate 13-acetate (PMA) (100 ng/ml) and incubated for 45 min at 37°C in 5% CO2. The reaction was stopped with 10 μl of 1 N NaOH, and the reactive wells were read in a microplate reader (Bio-Rad) with a 595-nm filter. Results are expressed as nanomoles of H2O2 released by 106 cells in 30 min.
Detection of O2− released by resident peritoneal cells.
Superoxide anion was quantitatively determined by nitroblue tetrazolium reduction. Peritoneal cells (4 × 106) were incubated in the dark for 30 min at 37°C with 5% CO2 in the presence of nitroblue tetrazolium (0.1%) with or without PMA at an optimal final concentration of 100 ng/ml. The reaction was stopped with 0.4 ml of 0.1 N HCl. Cells were centrifuged, and insoluble formazan was extracted twice with 1 ml of 1,4-dioxane. Optical densities in supernatants were determined at 560 nm, and results are expressed as the percentage of optical densities developed in control tubes.
Statistical evaluation.
Data from these studies were analyzed by one-way analysis of variance. Results giving P values of ≤0.05 were considered significantly different.
RESULTS
Food consumption, body weight, body weight gain, and fumonisin consumption.
Daily observations for 90 days did not indicate detectable alterations in the general state of any of the animals. The average food consumptions until the 30th, 60th, and 90th days were 36.8, 40.2, and 41.5 g, respectively, for control group animals, while the average consumptions for rats fed FB1 were 35.9, 39.8, and 34.3 g, respectively. Significant differences in food consumption, body weight, and body weight gain at days 30 and 60 were not observed. On day 90, decreases in food consumption (P ≤ 0.05), in weight (P ≤ 0.001), and in weight gain (P ≤ 0.001) were detected in the group fed FB1 with respect to the control group. The total average FB1 consumptions on days 30, 60, and 90 were 319, 544, and 810 mg of FB1/kg of body weight, respectively.
Examination of tissues.
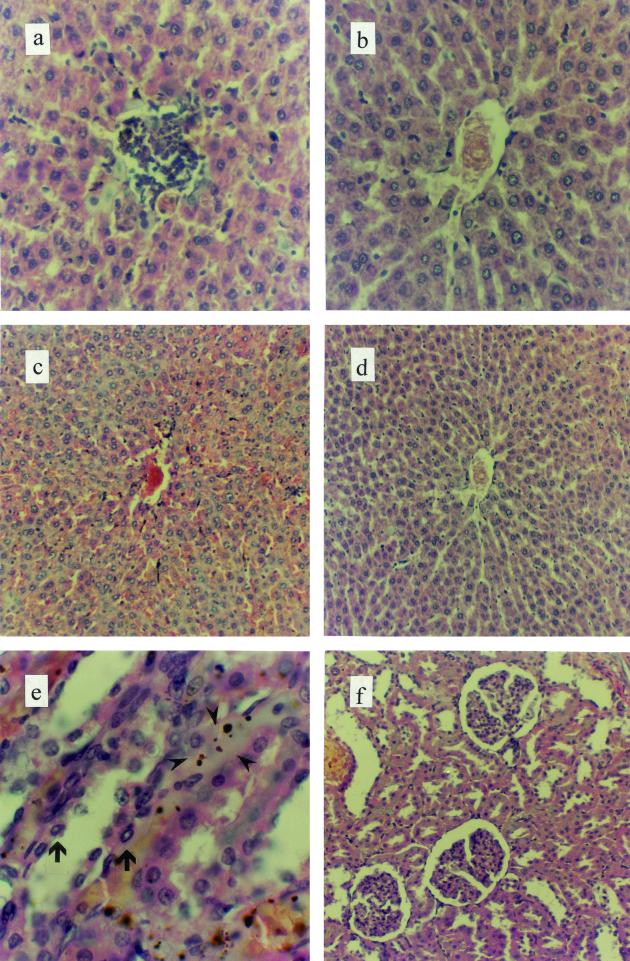
In the histopathologic examination the tissues of organs showed little modification in the cell structures of lungs and esophagus in FB1-fed animals compared with controls. On the other hand, in liver samples of FB1-fed rats, a perivascular histiocytic infiltrate (Fig. 1a) and an increased number of Kupffer cells and changes in the normal structure (Fig. 1c) in comparison with control animals (Fig. 1b and d, respectively) were detected. In the kidneys of rats fed FB1, apoptotic bodies and necrotic alterations in tubular epithelial cells (Fig. 1e), an increase in the capsular space (Fig. 1f), and the presence of proteinogenous material in the tubular lumen (lights) were observed. These kidney alterations were not detected in control rats. In addition, the microscopic examination showed an increase in the average number of mitotic cells in the base of the crypt (Fig. 2a) and a major lymphocytic infiltrate (Fig. 2b) in the small intestine in rats fed FB1.
FIG. 1.
Hematoxylin-and-eosin-stained sections of rat organs exposed to a diet containing 100 ppm of FB1 or a diet without FB1 (control) for 90 days. In the livers of animals fed FB1, a perivascular histiocytic infiltrate (a) with respect to control rats (b) and an increased number of Kupffer cells (c) compared to those of control rats (d) were the main alterations found. Histological findings in the kidney included apoptosis (arrows) and necrosis (arrowheads) of tubular epithelial cells (e), and increased capsular space (f) was also found. Magnifications, ×200 (a and b), ×100 (c, d, and f), and ×400 (e).
FIG. 2.
Hematoxylin-and-eosin-stained sections of rat organs exposed to a diet containing 100 ppm of FB1 for 90 days. In the small intestine, an increased number of mitotic cells (arrows) (a) and lymphocytic infiltrate (b) were present. Magnification, ×400 (a) and ×100 (b).
Serum biochemical measurements.
The data obtained from the biochemical profile are shown in Table 1. In the sera of animals fed FB1, an increase of ALP activity (P ≤ 0.001) and a decrease of TG levels (P ≤ 0.05) in comparison with control rats were observed No significant changes in Chol or Ca levels or in ALT, AST, and GGT activities were observed.
TABLE 1.
Serum parameters for rats (n = 6) on day 90
| Rats | Mean (SEM) level
|
||||||
|---|---|---|---|---|---|---|---|
| Chol (mg/dl) | TGs (mg/dl) | Ca (mg/dl) | AST (kata/liter) | ALT (kat/liter) | GGT (kat/liter) | ALP (kat/liter) | |
| Controlb | 73.50 (3.22) | 157.50 (15.82) | 11.37 (0.13) | 2.10 (0.19) | 0.65 (0.02) | 0.09 (0.02) | 2.64 (0.13) |
| FB1c | 71.67 (6.44) | 103.00d (15.62) | 11.37 (0.08) | 2.33 (0.19) | 0.92 (0.11) | 0.09 (0.01) | 4.53e (0.53) |
One katal catalyzes 1 mol of product/s under defined conditions.
Rats fed control diet for 90 days.
Rats fed diet with FB1 for 90 days.
P ≤ 0.05.
P ≤ 0.001.
Mitogenic responses of SMCs.
To examine the effects produced in the immunologic system by the subchronic FB1 intoxication, SMCs from control rats and from those fed FB1 in the basal state and in the presence of ConA and LPS were cultured. No significant differences were observed in [3H]thymidine uptake by SMCs in the basal state or in the presence of ConA or LPS between 72 and 96 h of culture. In in vitro assays, there were no important changes in the [3H]thymidine uptake when SMCs of normal rats were cultured with FB1 at 10 μM (7.21 μg/ml) in comparison to the basal proliferation or when they were cultured with FB1 at 10 μM (7.21 μg/ml) plus ConA at 10 μg/ml, in contrast to SMCs cultured with ConA at 10 μg/ml (data not shown).
Cytokine measurement.
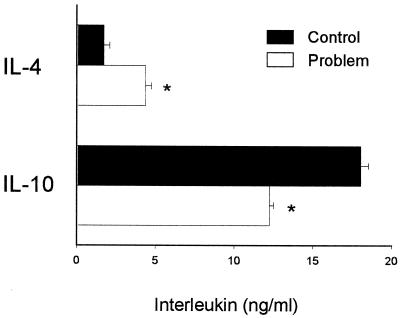
After 72 h of culture, supernatants of SMCs from animals fed FB1 had significantly higher concentrations of IL-4 (P ≤ 0.01) and lower concentrations of IL-10 (P ≤ 0.01) than controls (Fig. 3). There were no alterations in IL-2 levels produced by cells from FB1-fed rats in comparison with controls (data not shown). In in vitro assays, there were no changes in the levels of IL-2, IL-4, and IL-10 produced by SMCs of normal rats in the presence of FB1 (10 μM) with respect to controls.
FIG. 3.
Interleukins released by SMCs obtained from rats fed a control diet or a diet with FB1 (Problem). Cytokine levels in supernatants of cells cultured for 72 h after recovery from spleens were determined by enzyme-linked immunosorbent assay. Error bars indicate standard errors. *, P ≤ 0.01.
H2O2 and O2− released by resident peritoneal cells.
The levels of H2O2 produced by adherent peritoneal cells and the levels of anion superoxide produced by total peritoneal cells in the basal state and in the presence of PMA were quantified. The levels of H2O2 found are shown in Fig. 4. The peritoneal cells of animals fed FB1 produced significantly lower levels of H2O2 (P ≤ 0.01) than controls stimulated with PMA, while there were no differences in H2O2 levels produced in the basal state (Fig. 4A). In in vitro assays, adherent peritoneal cells from normal animals produced significantly lower concentrations of H2O2 (P ≤ 0.01) in the presence of FB1 (10 μM) and when stimulated by PMA than controls (Fig. 4B). There were no changes in anion superoxide production in the basal state or in the presence of PMA (data not shown).
FIG. 4.
H2O2 released by adherent peritoneal cells. Macrophages were incubated with or without PMA as a stimulant. Results are expressed as mean (standard error) nanomoles of H2O2 released by 106 cells in 30 min. (A) In vivo exposure to FB1 (n = 6 rats). Control, rats fed control diet for 90 days; Problem, rats fed diet with FB1 for 90 days. (B) In vitro exposure to FB1. For in vitro assays a pool of peritoneal cells from four normal rats was used. Cells were incubated with (Problem) or without (Control) 10 μM FB1. *, P ≤ 0.05; **, P ≤ 0.01.
DISCUSSION
In this study we have detected immunobiological alterations produced by ingestion of FB1 in a model of experimental subchronic mycotoxicosis in rats. In this model, the total ingestion was 303 mg of FB1 during 90 days, producing significant decreases in food consumption (17.4%), body weight (17.9%), and body weight gain (125.7%). Under similar experimental conditions in a murine model, our group observed that with a total ingestion of 7.37 mg of FB1 there were also losses in weight and in weight gain in the animals; however, in contrast to what happened in rats, the food consumption was higher (5). On the other hand, Voss et al. have reported a decrease in food consumption, body weight, and body weight gain in a model of rats that consumed 228 ppm of FB1 plus 58 ppm of FB2 plus 17 ppm of FB3 for a 3-week period (43). These observations suggest that FB1 can modify these parameters in different ways, depending on the animal model and the experimental scheme used.
In histopathologic examination alterations similar to those described by other authors were found, indicating that the liver and kidney are the principal target organs for FB1 action in rats. Furthermore, the lymphocytic infiltrate and increased average number of mitotic cells found by the crypt base in the intestine were present in all samples of animals fed FB1. Despite the fact that the small intestine is not one of the organs most affected by this mycotoxin, it is exposed to the same FB1 concentrations via oral administration. FB1 is able to have toxic activity on the intestinal cell by interference in the lipid metabolism (13), causing alteration of the cellular cycle (29) and increasing the cell number in different phases of mitosis (Fig. 2a). These findings would be related to a major susceptibility to infections by pathogens that enter via the oral route (39).
The level of serum TGs is influenced by fats introduced in the diet and endogenous synthesis in the liver and intestine (2). In this work, a decrease in the TG concentration in animals fed FB1 was observed (Table 1). Bondy et al. (4) have reported a similar finding in a study of acute toxicity in rats in which the food consumption and therefore the fats in the diet were diminished for only a short time. On the other hand, Enongene et al. (13) have reported alterations in lipid metabolism in the epithelial cells of the small intestine and hepatocytes. These results indicate that FB1 could be a cause of the decreased levels of TGs in serum in our model, interfering in the biosynthesis of endogenous TGs.
Among the parameters studied in the biochemical profile, an increase of ALP activity was found. Although the liver is the major source of this enzyme, in some cases in which the intestinal metabolism is stimulated, the intestinal isoenzyme could represent an important factor (21). A similar effect is obtained due to cellular alterations in the proximal convoluted tubules of the kidney, which may contribute to the total serum ALP activity (21). These results are related to the histopathologic findings in the kidney (Fig. 1e).
The failure to observe changes in SMC proliferation in animals fed FB1 (in the basal state or stimulated), as well as in the proliferation of normal SMCs exposed in vitro to FB1, is due to the mycotoxin concentration used. These results are related to the observations of Tryphonas et al. (39) that the daily ingestion of 25 mg of FB1/kg of body weight/day for 14 days did not produce changes in the proliferative response of rat lymphocytes. Charoenpornsook et al. (6), using bovine peripheral blood mononuclear cells, have observed a 50% decrease of proliferation in the presence of ConA when the cells were exposed to 35 μg of FB1 per ml. Taking into account the pharmacokinetic data reported by Martinez-Larranaga et al. (27), in our experimental model the major FB1 concentrations that could arise in blood would be 5 to 10 μg/ml. Even if there are differences among species, higher FB1 concentrations in rats than the one used in this work (7.21 μg/ml) would be needed to produce alterations in the normal blostomytogenic response of lymphocytes.
Little is known about the function of interleukins in a mycotoxicosis produced by fumonisins. In our work, higher concentrations of IL-4 and lower concentrations of IL-10 in supernatants of SMCs in rats fed with FB1 were found with respect to controls (Fig. 3). This increase of IL-4 could be stimulated by the presence of FB1 and/or the accumulation of sphingoid bases (sphingosine) in the intracellular space by means of an unknown mechanism (28). Therefore, the ingestion of FB1 during a subchronic period could produce a break in the balance of Th1 and Th2 subsets. In models of chronic FB1 intoxication, the main expression of some interleukins could be related to the evasion of tumor cells from immunologic surveillance (24). Furthermore, it was determined that among the functions of IL-10, this cytokine could act as a costimulator for the growth of mature thymocytes. It also functions as a cytotoxic-T-cell differentiation factor, promoting a higher number of IL-2-activated cytotoxic-T-lymphocyte precursors to proliferate and differentiate into cytotoxic effector cells (7). It has also been suggested that IL-10 is an essential immunoregulator of the intestinal tract and that the generalized bowel inflammation in IL-10-deficient animals is due to uncontrolled immune responses stimulated by enteric antigens (23). The decrease of IL-10 found in animals fed FB1 could contribute to the alterations observed in the small intestine. On the other hand, the absence of modifications in the IL-2 levels in these animals in comparison with controls would be related to the results obtained on the proliferation of SMCs in the presence of ConA.
The presence of some Th2 profile cytokines could have also been modulating the macrophage function (32). The hydrogen peroxide and anion superoxide produced by these cells have an important role in the host defense against tumors and microorganisms. In our experimental model, peritoneal macrophages exposed in vivo and in vitro to FB1 produced less hydrogen peroxide (Fig. 4); however, alterations in the production of anion superoxide were not found. These results suggest that FB1 can have immunosuppressive effects on some of the macrophage immunologic mechanisms, diminishing their cytotoxic capacity, which would be related to a lower antitumor activity.
The results obtained in this work indicated that FB1 has the liver and kidney as principal target organs for subchronic toxicity in rats. Further, in this model the small intestine is clearly affected. With the doses used, FB1 is able to produce a modification of the excretion of interleukins, acting on macrophage function. A more extensive study on the accumulation of sphingoid bases in the immune cell system and its functionality would be able to clarify the mechanisms acting in the pathogenesis of this intoxication.
Acknowledgments
This work was supported by Agencia Nacional de Ciencia y Tecnología grant FONCYT-PICT 09-03688.
REFERENCES
- 1.Azcona-Olivera, J. I., Y-L. Ouyang, R. L. Warner, J. E. Linz, and J. J. Pestka. 1995. Effects of vomitoxin (deoxynivalenol) and cycloheximide on IL-2, 4, 5 and 6 secretion and RNA levels in murine CD4+ cells. Food Chem. Toxicol. 33:433–441. [DOI] [PubMed] [Google Scholar]
- 2.Bachorick, P. S., R. I. Levy, and B. M. Rifkind. 1993. Lípidos y dislipoproteinemias, p.195–221. In J. B. Henry (ed.), Diagnóstico y tratamiento clínicos por el laboratorio, 19th ed. W. B. Saunders Co., Philadelphia, Pa.
- 3.Blackwell, B. A., O. E. Edwards, A. Fruchier, J. W. ApSimon, and J. D. Miller. 1996. NMR structural studies of fumonisin B1 and related compounds from Fusarium moniliforme. Adv. Exp. Med. Biol. 392:75–91. [DOI] [PubMed] [Google Scholar]
- 4.Bondy, G., M. Barker, R. Mueller, S. Fernie, J. D. Miller, C. Armstrong, S. L. Hierlihy, P. Rowsell, and C. Suzuki. 1996. Fumonisin B1 toxicity in male Sprague-Dawley rats. Adv. Exp. Med. Biol. 392:251–264. [DOI] [PubMed] [Google Scholar]
- 5.Casado, J. M., M. Theumer, D. T. Masih, S. Chulze, and H. R. Rubinstein. 2000. Experimental subchronic mycotoxicoses in mice. Individual and combined effects of dietary exposure to fumonisins and aflatoxin B1. Food Chem. Toxicol. 39:579–586. [DOI] [PubMed] [Google Scholar]
- 6.Charoenpornsook, K., J. L. Fitzpatrick, and J. E. Smith. 1998. The effects of four mycotoxins on the mitogen stimulated proliferation of bovine peripheral blood mononuclear cells in vitro. Mycopathologia 143:105–111. [DOI] [PubMed] [Google Scholar]
- 7.Chen, W. F., and A. Zlotnik. 1991. IL-10: a novel cytotoxic T cell differentiation factor. J. Immunol. 147:528–534. [PubMed] [Google Scholar]
- 8.Choi, C. Y., H. Nakajima-Adachi, S. Kaminogawa, and Y. Sugita-Konishi. 2000. Nivalenol inhibits total and antigen-specific IgE production in mice. Toxicol. Appl. Pharmacol. 165:94–98. [DOI] [PubMed] [Google Scholar]
- 9.Chulze, S. N., M. L. Ramirez, A. Torres, and J. F. Leslie. 2000. Genetic variation in Fusarium section Liseola from no-till maize in Argentina. Appl. Environ. Microbiol. 66:5312–5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colvin, B. M., and L. R. Harrison. 1992. Fumonisin-induced pulmonary edema and hydrothorax in swine. Mycopathologia 117:79–82. [DOI] [PubMed] [Google Scholar]
- 11.Dombrink-Kurtzman, M. A., R. Gomez-Flores, and R. J. Weber. 2000. Activation of rat splenic macrophage and lymphocyte functions by fumonisin B(1). Immunopharmacology 49:401–409. [DOI] [PubMed] [Google Scholar]
- 12.Dombrink-Kurtzman, M. A., T. Javed, G. A. Bennet, J. L. Richard, L. M. Cote, and W. B. Buck. 1993. Lymphocyte cytotoxicity and erythrocytic abnormalities induced in broiler chicks by fumonisin B1 and B2 and moniliformin from Fusarium proliferatum. Mycopathologia. 124:47–54. [DOI] [PubMed] [Google Scholar]
- 13.Enongene, E. N., R. P. Sharma, N. Bhandari, K. A. Voss, and R. T. Riley. 2000. Disruption of sphingolipid metabolism in small intestines, liver and kidney of mice dosed subcutaneously with fumonisin B(1). Food Chem. Toxicol. 38:793–799. [DOI] [PubMed] [Google Scholar]
- 14.Etcheverry, M., A. Nesci, G. Barros, and S. Chulze. 1999. Occurrence of Aspergillus section flavi and aflatoxin B1 in corn genotypes and corn meal in Argentina. Mycopathologia 147:37–41. [DOI] [PubMed] [Google Scholar]
- 15.Gelderblom, W. C., M. E. Cawood, S. D. Snyman, and W. F. Marasas. 1994. Fumonisin B1 dosimetry in relation to cancer initiation in rat liver. Carcinogenesis 15:209–214. [DOI] [PubMed] [Google Scholar]
- 16.Gonzales, H. H., E. J. Martinez, A. M. Pacin, S. L. Pacin, S. L. Resnik, and E. W. Sydenham. 1999. Natural co-occurrence of fumonisins, deoxynivalenol, zearalenone and aflatoxins in field trial corn in Argentina. Food Addit. Contam. 16:565–569. [DOI] [PubMed] [Google Scholar]
- 17.Gutema, T., C. Munimbazi, and L. B. Bullerman. 2000. Occurence of fumonisins and moniliformin in corn and corn-based food products of U.S. origin. J. Food Prot. 63:1732–1737. [DOI] [PubMed] [Google Scholar]
- 18.Hengstler, J. G., B. Van de Burg, P. Steinberg, and F. Oesch. 1999. Interspecies differences in cancer susceptibility and toxicity. Drug Metab. Rev. 31:917–970. [DOI] [PubMed] [Google Scholar]
- 19.Hennigen, M. R., S. Sanchez, N. M. Di Benedetto, A. Longhi, J. E. Torroba, and L. M. Valente Soares. 2000. Fumonisin levels in commercial corn products in Buenos Aires, Argentina. Food Addit. Contamin. 17:55–58. [DOI] [PubMed] [Google Scholar]
- 20.Ji, G. E., S. Y. Park, S. S. Wong, and J. J. Pestka. 1998. Modulation of nitric oxide, hydrogen peroxide and cytokine production in a clonal macrophage model by the tricothecene vomitoxin (deoxynivalenol). Toxicology 125:203–214. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan, M. M. 1993. Laboratory test, p.108–144. In L. Schiff and E. R. Schiff (ed.), Diseases of the liver, 7th ed. Lippincott, Philadelphia, Pa.
- 22.Kisaki, T., S. Kobayashi, K. Ogasawra, and N. K. Day. 1991. Immune suppression induced by protoscoleces of E. multilocularis in mice. Evidence for the presence of CD8dull suppressor cells in spleens of mice intraperitoneally infected with E. multilocularis. J. Immunol. 147:1659–1663. [PubMed] [Google Scholar]
- 23.Kuhn, R., J. Lohler, D. Rennick, K. Rajewsky, and W. Muller. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263–274. [DOI] [PubMed] [Google Scholar]
- 24.Liu, J., Z. Tian, and R. Sun. 1998. The predominant expression of Th2 type cytokines in human tumor cells. Chung. Hua. Chung. Liu. Tsa. Chih. 20:105–107. [PubMed] [Google Scholar]
- 25.Machinski, M., Jr., and L. M. Soares. 2000. Fumonisins B1 and B2 in Brazilian corn-based food products. Food Addit. Contam. 17:875–879. [DOI] [PubMed] [Google Scholar]
- 26.Marasas, W. F., K. Jaskiewicz, F. S. Venter, and D. J. Schalkwyk. 1988. Fusarium moniliforme contamination of maize in oesophageal cancer areas in Transkei. S. Afr. Med. J. 74:110–114. [PubMed] [Google Scholar]
- 27.Martinez-Larranaga, M. R., A. Anadon, M. J. Diaz, M. L. Fernández Cruz, M. A. Martinez, M. T. Frejo, M. Martinez, R. Fernandez, R. M. Anton, M. E. Morales, and M. Tafur. 1999. Toxicokinetics and oral bioavailability of fumonisin B1. Vet. Hum. Toxicol. 41:357–362. [PubMed] [Google Scholar]
- 28.Martinova, E. A. 1997. Influence of sphingolipids on T lymphocyte activation. Biochemistry (Moscow). 63:102–110. [PubMed] [Google Scholar]
- 29.Merrill, A. H., E. M. Schmelz, D. L. Dillehay, S. Spiegel, J. A. Shayman, J. J. Schroeder, R. T. Riley, K. A. Voss, and E. Wang. 1997. Sphingolipids—the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol. Appl. Pharmacol. 142:208–225. [DOI] [PubMed] [Google Scholar]
- 30.Merril, A. H., E. Wang, T. R. Vales, E. R. Smith, J. J. Schroeder, D. S. Menaldino, C. Alexander, H. M. Crane, J. Xia, D. C. Liotta, F. I. Meredith, and R. T. Riley. 1996. Fumonisin toxicity and sphingolipid biosynthesis. Adv. Exp. Med. Biol. 392:297–306. [DOI] [PubMed] [Google Scholar]
- 31.Moon, E. Y., D. K. Rhee, and S. Pyo. 1999. Inhibition of various functions in murine peritoneal macrophages by aflatoxin B1 exposure in vivo. Int. J. Immunopharmacol. 21:47–58. [DOI] [PubMed] [Google Scholar]
- 32.Nemoto, Y. T. Otsuka, H. Niiro, K. Izuhara, K. Yamahoka, H. Nakashima, and Y. Niho. 1999. Differential effects of interleukin-4 and interleukin-10 on nitric oxide production by murine macrophages. Inflamm. Res. 48:643–650. [DOI] [PubMed] [Google Scholar]
- 33.Pitt, J. I. 2000. Toxigenic fungi and mycotoxins. Br. Med. Bull. 56:184–192. [DOI] [PubMed] [Google Scholar]
- 34.Ross, P. F., A. E. Ledet, D. L. Owens, L. G. Rice, H. A. Nelson, G. D. Osweiler, and T. M. Wilson. 1993. Experimental equine leukoencephalomalacia, toxic hepatosis, and encephalopathy caused by corn naturally contaminated with fumonisins. J. Vet. Diagn. Investig. 5:69–74. [DOI] [PubMed] [Google Scholar]
- 35.Sabder, B., I. Hoiden, U. Andersson, E. Moller, and J. S. Abrams. 1993. Similar frequencies and kinetics of cytokine producing cells in murine peripheral blood and spleen. J. Immunol. Methods 166:201–214. [DOI] [PubMed] [Google Scholar]
- 36.Shephard, G. S., E. W. Sydenham, P. G. Thiel, and W. C. A. Gelderblom. 1990. Quantitative determination of fumonisins B1 and B2 by high-performance liquid chromatography with fluorescence detection. J. Liquid Chromatogr. 13:2077–2087. [Google Scholar]
- 37.Shim, W. B., and C. P. Woloshuk. 1999. Nitrogen repression of fumonisin B1 biosynthesis in Gibberella fujikuroi. FEMS Microbiol. Lett. 177:109–116. [DOI] [PubMed] [Google Scholar]
- 38.Tolleson, W. H., K. L. Dooley, W. G. Sheldon, J. D. Thurman, T. J. Bucci, and P. C. Howard. 1996. The mycotoxin fumonisin induces apoptosis in cultured human cells and in livers and kidneys of rats. Adv. Exp. Med. Biol. 392:237–250. [DOI] [PubMed] [Google Scholar]
- 39.Tryphonas, H., G. Bondy, J. D. Miller, F, Lacroix, M. Hodgen, P. McGuire, S. Fernie, D. Miller, and S. Hayward. 1997. Effects of fumonisin B1 on the immune system of Sprague-Dawley rats following a 14-day oral (gavage) exposure. Fundam. Appl. Toxicol. 39:53–59. [PubMed] [Google Scholar]
- 40.van der Westhuizen, L., G. S. Shephard, S. D. Snyman, S. Abel, S. Swanevelder, and W. C. Gelderblom. 1998. Inhibition of sphingolipid biosynthesis in rat primary hepatocyte cultures by fumonisin B1 and other structurally related compounds. Food Chem. Toxicol. 36:497–503. [DOI] [PubMed] [Google Scholar]
- 41.Voss, K. A., R. T. Riley, C. W. Bacon, W. J. Chamberlain, and W. P. Norred. 1996. Subchronic toxic effects of Fusarium moniliforme and fumonisin B1 in rats and mice. Nat. Toxins 4:16–23. [DOI] [PubMed] [Google Scholar]
- 42.Voss, K. A., R. D. Plattner, C. W. Bacon, and W. P. Norred. 1990. Comparative studies of hepatotoxicity and fumonisin B1 and B2 content of water and chloroform/methanol extracts of Fusarium moniliforme strain MRC 826 culture material. Mycopathologia 112:81–92. [DOI] [PubMed] [Google Scholar]
- 43.Voss, K. A., R. D. Plattner, R. T. Riley, F. I. Meredith, and W. P. Norred. 1998. In vivo effects of fumonisin B1 nonproducing Fusarium moniliforme isolates are similar: fumonisins B2 and B3 cause hepato- and nephrotoxicity in rats. Mycopathologia 141:45–58. [DOI] [PubMed] [Google Scholar]
- 44.Yamada, A., T. Kataoka, and K. Nagai. 2000. The fungal metabolite gliotoxin: immunosuppressive activity on CTL-mediated cytotoxicity. Immunol. Lett. 71:27–32. [DOI] [PubMed] [Google Scholar]