Abstract
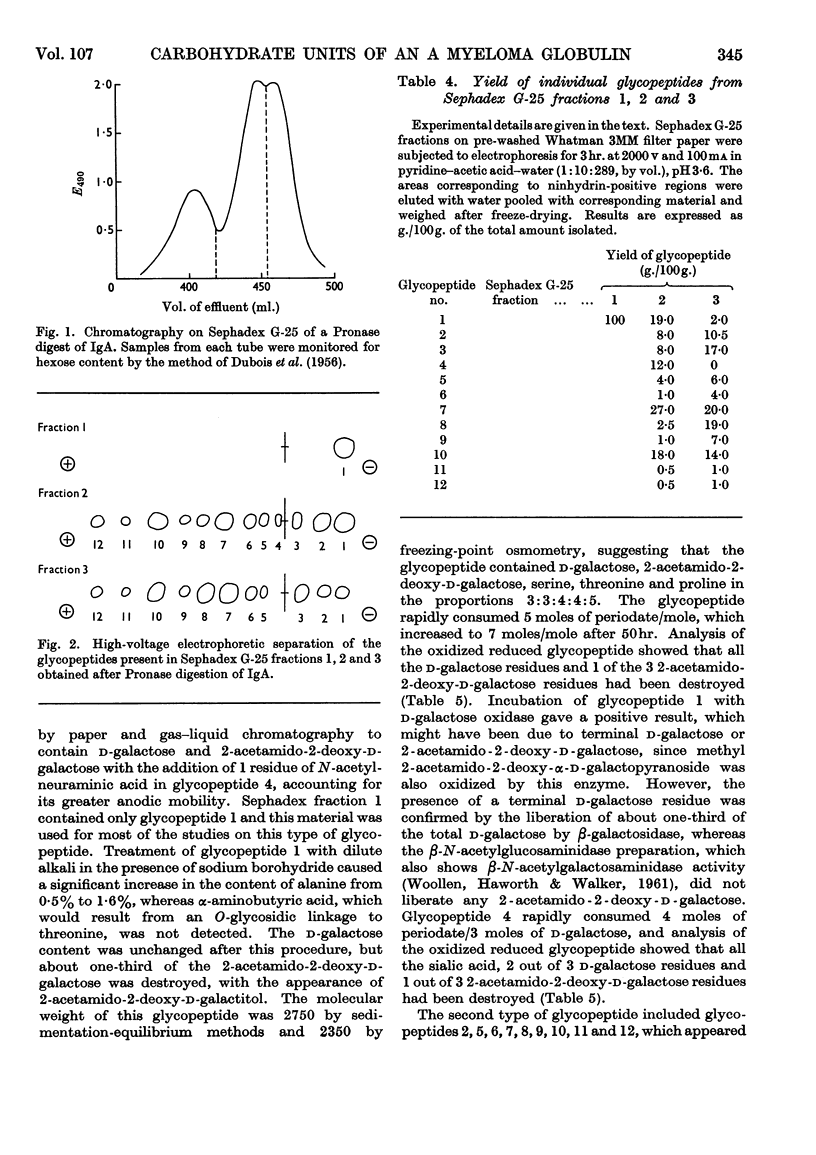
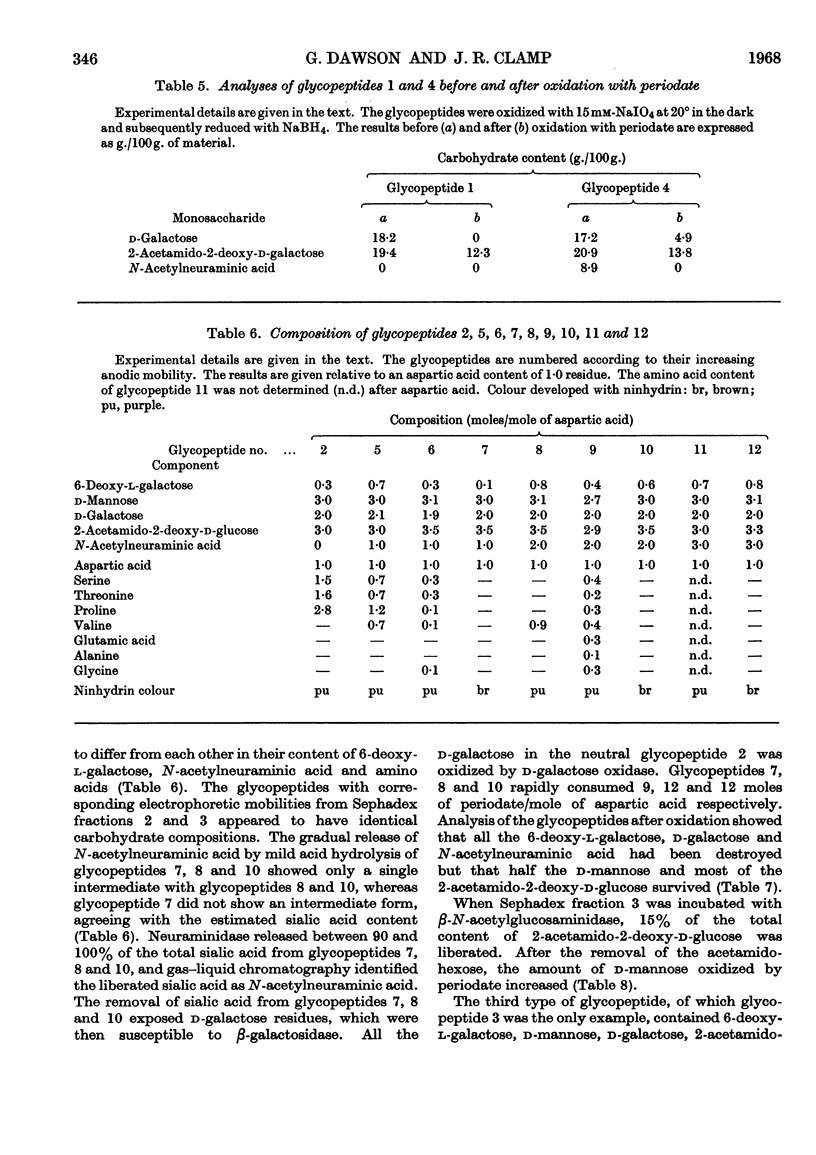
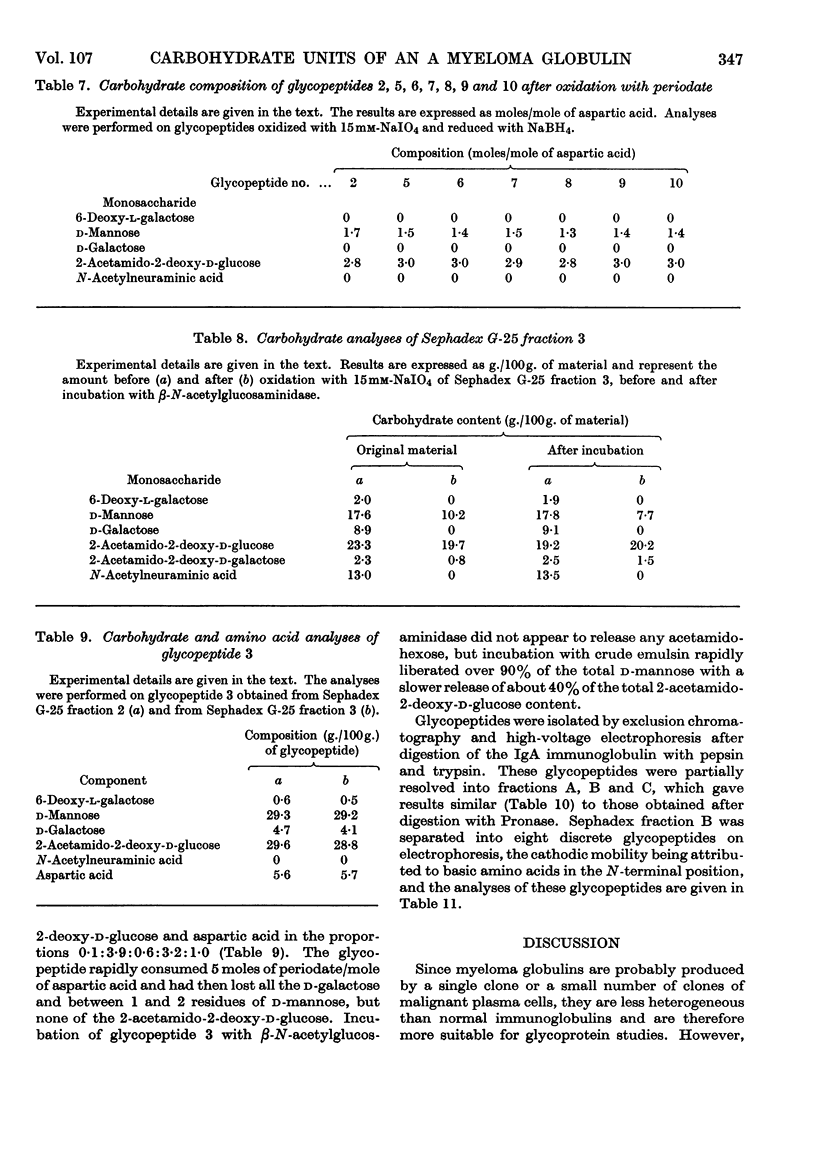
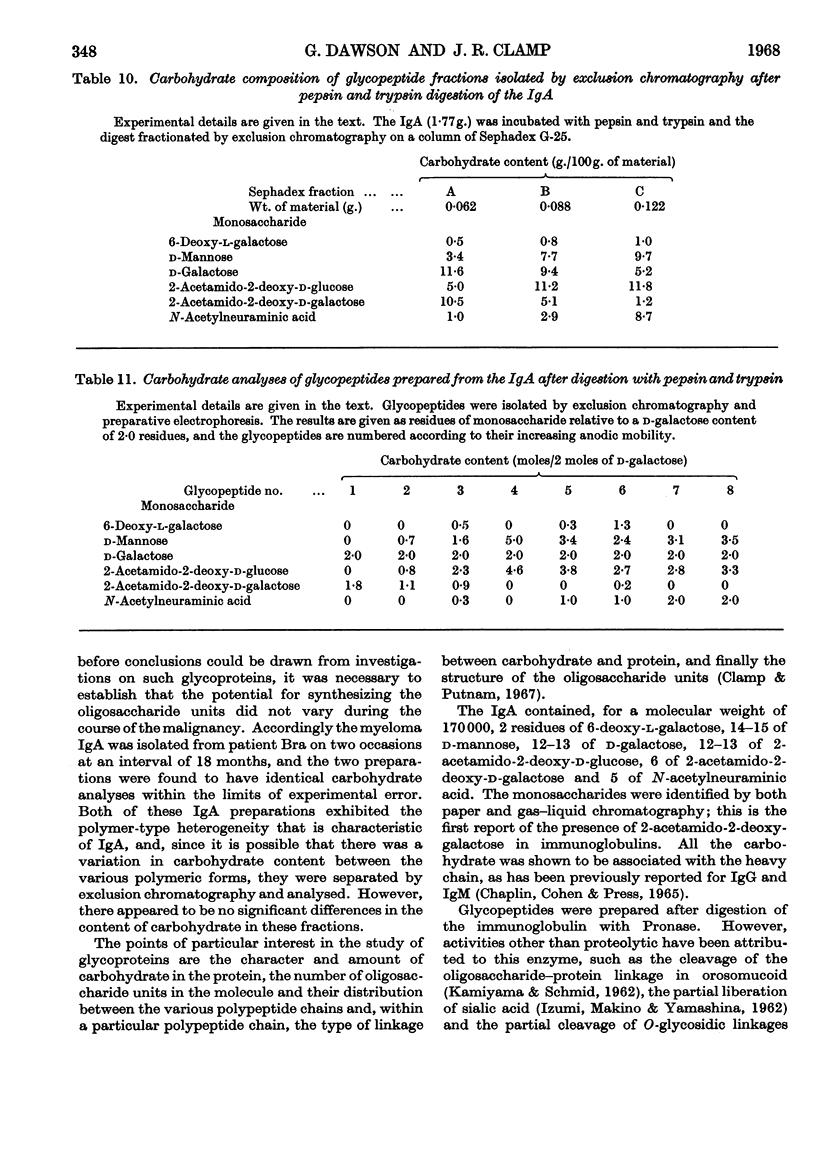
The carbohydrate content of an A myeloma globulin was investigated. The carbohydrate content was found to be unchanged when the protein was isolated from the patient over a period of 18 months. The various polymeric forms of the protein contained similar proportions of carbohydrate. The A myeloma globulin contained approx. 2 residues of 6-deoxy-l-galactose (l-fucose), 14–15 of d-mannose, 12–13 of d-galactose, 12–13 of 2-acetamido-2-deoxy-d-glucose (N-acetyl-d-glucosamine), 6 of 2-acetamido-2-deoxy-d-galactose (N-acetyl-d-galactosamine) and 5 of N-acetylneuraminic acid (sialic acid), and these were distributed between six oligosaccharide units all of which were present on the heavy polypeptide chains. The oligosaccharide units showed two kinds of heterogeneity, which have been termed central and peripheral. Central heterogeneity was shown by the presence of three completely different core units, which had the following compositions: (1) 3 residues of d-galactose and 3 of 2-acetamido-2-deoxy-d-galactose, joined to protein by an O-glycosidic linkage between acetamidohexose and serine; (2) 3 residues of d-mannose, 2 of d-galactose and 3 of 2-acetamido-2-deoxy-d-glucose, joined to protein by an N-glycosidic linkage between acetamidohexose and aspartic acid; (3) 4 residues of d-mannose and 3 of 2-acetamido-2-deoxy-d-glucose with a linkage similar to that in (2). The core oligosaccharide units showed peripheral heterogeneity in the attachment of 6-deoxy-l-galactose, 2-acetamido-2-deoxy-d-glucose and N-acetylneuraminic acid. Tentative structures are proposed for these various types of oligosaccharide unit. Glycopeptides were isolated in which the sialic acid content exceeded that of d-galactose. Explanations are given for the electrophoretic mobility and staining characteristics of the various glycopeptides.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKER S. A., PARDOE G. I., STACEY M., HOPTON J. W. Sequential enzyme induction a new approach to the structure of complex mucoproteins. Nature. 1963 Jan 19;197:231–233. doi: 10.1038/197231a0. [DOI] [PubMed] [Google Scholar]
- BLIX G. The linkage between hexosamine and amino acids in ovine submaxillary mucin. Ann N Y Acad Sci. 1963 Mar 30;106:164–167. doi: 10.1111/j.1749-6632.1963.tb16635.x. [DOI] [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- CARUBELLI R., BHAVANANDAN P., GOTTSCHALK A. STUDIES ON GLYCOPROTEINS. XI. THE O-GLYCOSIDIC LINKAGE OF N-ACETYLGALACTOSAMINE TO SERYL AND THREONYL RESIDUES IN OVINE SUBMAXILLARY GLAND GLYCOPROTEIN. Biochim Biophys Acta. 1965 Mar 1;101:67–82. doi: 10.1016/0926-6534(65)90031-x. [DOI] [PubMed] [Google Scholar]
- CHAPLIN H., COHEN S., PRESS E. M. PREPARATION AND PROPERTIES OF THE PEPTIDE CHAINS OF NORMAL HUMAN 19 S GAMMA-GLOBULIN (IGM). Biochem J. 1965 Apr;95:256–261. doi: 10.1042/bj0950256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHODIRKER W. B., TOMASI T. B., Jr GAMMA-GLOBULINS: QUANTITATIVE RELATIONSHIPS IN HUMAN SERUM AND NONVASCULAR FLUIDS. Science. 1963 Nov 22;142(3595):1080–1081. doi: 10.1126/science.142.3595.1080. [DOI] [PubMed] [Google Scholar]
- CLAMP J. R., PUTNAM F. W. THE CARBOHYDRATE PROSTHETIC GROUP OF HUMAN GAMMA-GLOBULIN. J Biol Chem. 1964 Oct;239:3233–3240. [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- Clamp J. R., Dawson G., Hough L. The simultaneous estimation of 6-deoxy-L-galactose (L-fucose), D-mannose, D-galactose, 2-acetamido-2-deoxy-D-glucose (N-acetyl-D-glucosamine) and N-acetylneuraminic acid (sialic acid) in glycopeptides and glycoproteins. Biochim Biophys Acta. 1967 Nov 28;148(2):342–349. doi: 10.1016/0304-4165(67)90129-8. [DOI] [PubMed] [Google Scholar]
- Clamp J. R., Putnam F. W. Glycopeptides of immunoglobulins. Investigations on IgA myeloma globulins. Biochem J. 1967 Apr;103(1):225–229. doi: 10.1042/bj1030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEUTSCH H. F. MOLECULAR TRANSFORMATIONS OF A GAMMA1-GLOBULIN OF HUMAN SERUM. J Mol Biol. 1963 Dec;7:662–671. doi: 10.1016/s0022-2836(63)80114-x. [DOI] [PubMed] [Google Scholar]
- EYLAR E. H., JEANLOZ R. W. Periodate oxidation of the alpha1 acid glycoprotein of human plasma. J Biol Chem. 1962 Apr;237:1021–1025. [PubMed] [Google Scholar]
- EYLAR E. H. The carbohydrate-protein linkage in the alpha1-glycoprotein of human plasma. Biochem Biophys Res Commun. 1962 Jul 3;8:195–199. doi: 10.1016/0006-291x(62)90262-0. [DOI] [PubMed] [Google Scholar]
- Elson L. A., Morgan W. T. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27(6):1824–1828. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY F. L., LAWRENCE M. E. QUANTITATIVE DETERMINATION OF 6.6 S GAMMA-GLOBULINS, BETA-2A-GLOBULINS AND GAMMA-1-MACROGLOBULINS IN HUMAN SERUM. J Immunol. 1963 Nov;91:597–603. [PubMed] [Google Scholar]
- FRANKLIN E. C. STRUCTURAL STUDIES OF HUMAN 7S GAMMA-GLOBULIN (G IMMUNOGLOBULIN). FURTHER OBSERVATIONS OF A NATURALLY OCCURRING PROTEIN RELATED TO THE CRYSTALLIZABLE (FAST) FRAGMENT. J Exp Med. 1964 Nov 1;120:691–709. doi: 10.1084/jem.120.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN E. C. Structural units of human 7S gamma globulin. J Clin Invest. 1960 Dec;39:1933–1941. doi: 10.1172/JCI104218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO Y., TSUIKI S., NISIZAWA K., PIGMAN W. Action of proteolytic enzymes on purified bovine submaxillary mucin. Ann N Y Acad Sci. 1963 Mar 30;106:233–246. doi: 10.1111/j.1749-6632.1963.tb16641.x. [DOI] [PubMed] [Google Scholar]
- HEREMANS J. F. Immunochemical studies on protein pathology. The immunoglobulin concept. Clin Chim Acta. 1959 Sep;4:639–646. doi: 10.1016/0009-8981(59)90004-x. [DOI] [PubMed] [Google Scholar]
- IZUMI K., MAKINO M., YAMASHINA I. Isolation and analysis of a carbohydrate-amino acid complex from an enzymic digest of alpha1-acid glycoprotein of human plasma. Biochim Biophys Acta. 1961 Jun 10;50:196–197. doi: 10.1016/0006-3002(61)91086-1. [DOI] [PubMed] [Google Scholar]
- JAMIESON G. A. STUDIES ON GLYCOPROTEINS. I. THE CARBOHYDRATE PORTION OF HUMAN CERULOPLASMIN. J Biol Chem. 1965 May;240:2019–2027. [PubMed] [Google Scholar]
- KAMIYAMA S., SCHMID K. Studies on the structure of alpha1-acid glycoprotein. II. Preparation and characterization of a glycopeptide fraction. Biochim Biophys Acta. 1962 Mar 26;58:80–87. doi: 10.1016/0006-3002(62)90819-3. [DOI] [PubMed] [Google Scholar]
- KUHN R. Les oligosaccharides du lait. Bull Soc Chim Biol (Paris) 1958;40(2-3):297–314. [PubMed] [Google Scholar]
- LAURELL C. B., LAURELL H., WALDENSTROM J. Glycoproteins in serum from patients with myeloma, macroglobulinemia and related conditions. Am J Med. 1957 Jan;22(1):24–36. doi: 10.1016/0002-9343(57)90335-2. [DOI] [PubMed] [Google Scholar]
- MARSHALL R. D., NEUBERGER A. CARBOHYDRATES IN PROTEIN. 8. THE ISOLATION OF 2-ACETAMIDO-1-(L-BETA-ASPARTAMIDO)-1,2-DIDEOXY-BETA-D-GLUCOSE FROM HEN'S EGG ABUMIN. Biochemistry. 1964 Oct;3:1596–1600. doi: 10.1021/bi00898a036. [DOI] [PubMed] [Google Scholar]
- MONTREUIL J., CHOSSON A., HAVEZ R., MULLET S. [Isolation of beta2A-globulins from human milk]. C R Seances Soc Biol Fil. 1960;154:732–736. [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J., KUNKEL H. G. The carbohydrate of gamma-globulin and myeloma proteins. J Exp Med. 1956 Aug 1;104(2):253–269. doi: 10.1084/jem.104.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F. Molecular size of gamma-A-immunoglobulin from bronchial secretions. Biochim Biophys Acta. 1966 May 12;120(1):172–173. doi: 10.1016/0926-6585(66)90292-5. [DOI] [PubMed] [Google Scholar]
- Miller F., Metzger H. Characterization of a human macroglobulin. II. Distribution of the disulfide bonds. J Biol Chem. 1965 Dec;240(12):4740–4745. [PubMed] [Google Scholar]
- Monsigny M., Montreuil J. Etude sur les glycoprotéides. Nature du point d'attache glycanne-protéine dans l'ovomucoïde. C R Acad Sci Hebd Seances Acad Sci D. 1966 Apr 18;262(16):1780–1783. [PubMed] [Google Scholar]
- Monsigny M., Montreuil J. Etudes sur les glycoprotéides. Mise en évidence d'une liaison de l'acide aspartique et de la glucosamine et d'une liaison O-thréonylglycosidique dans la lactotransferrine humaine. C R Acad Sci Hebd Seances Acad Sci D. 1966 Sep 19;263(12):893–895. [PubMed] [Google Scholar]
- Montreuil J., Adam-Chosson A., Spik G. Etude sur les glycoprotéides. IX. Etudes cinétiques de l'hydrolyse chimique des glycoprotéides. Application à l'ovomucoïde. Hypothèse de l'existence d'un schéma général de structure de la fraction glucidique des glycoprotéides d'origine animale. Bull Soc Chim Biol (Paris) 1965;47(10):1867–1880. [PubMed] [Google Scholar]
- Morgan W. T., Elson L. A. A colorimetric method for the determination of N-acetylglucosamine and N-acetylchrondrosamine. Biochem J. 1934;28(3):988–995. doi: 10.1042/bj0280988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REMINGTON J. S., VOSTI K. L., LIETZE A., ZIMMERMAN A. L. SERUM PROTEINS AND ANTIBODY ACTIVITY IN HUMAN NASAL SECRETIONS. J Clin Invest. 1964 Aug;43:1613–1624. doi: 10.1172/JCI105037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEVEAR J. W., SMITH E. L. Glycopeptides. I. Isolation and properties of glycopeptides from a fraction of human gamma-globulin. J Biol Chem. 1961 Feb;236:425–435. [PubMed] [Google Scholar]
- ROTHFUS J. A., SMITH E. L. Glycopeptides. IV. The periodate oxidation of glycopeptides from human gamma-globulin. J Biol Chem. 1963 Apr;238:1402–1410. [PubMed] [Google Scholar]
- SIMKIN J. L., SESHADRI H. S., SKINNER E. R. ACTION OF GLYCOSIDASES AND PERIODATE ON GUINEA PIG SERUM GLYCOPROTEINS. Nature. 1964 May 16;202:702–703. doi: 10.1038/202702b0. [DOI] [PubMed] [Google Scholar]
- SPIRO R. G. Studies on the monosaccharide sequence of the serum glycoprotein fetuin. J Biol Chem. 1962 Mar;237:646–652. [PubMed] [Google Scholar]
- Spiro R. G. Studies on the renal glomerular basement membrane. Nature of the carbohydrate units and their attachment to the peptide portion. J Biol Chem. 1967 Apr 25;242(8):1923–1932. [PubMed] [Google Scholar]
- Suzuki T., Deutsch H. F. Dissociation, reaggregation, and subunit structure studies of some human gamma-M-globulins. J Biol Chem. 1967 Jun 10;242(11):2725–2738. [PubMed] [Google Scholar]
- TOMASI T. B., Jr, TAN E. M., SOLOMON A., PRENDERGAST R. A. CHARACTERISTICS OF AN IMMUNE SYSTEM COMMON TO CERTAIN EXTERNAL SECRETIONS. J Exp Med. 1965 Jan 1;121:101–124. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WOOLLEN J. W., HEYWORTH R., WALKER P. G. Studies on glucosaminidase. 3. Testicular N-acetyl-beta-glucosaminidase and N-acetyl-beta-galactosaminidase. Biochem J. 1961 Jan;78:111–116. doi: 10.1042/bj0780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashina I., Makino M., Banì K., Kojima T. Polysaccharide-protein linkage in ovalbumin and orosomucoid (alpha-1-acid glycoprotein of human plasma). J Biochem. 1965 Aug;58(2):168–173. doi: 10.1093/oxfordjournals.jbchem.a128179. [DOI] [PubMed] [Google Scholar]


