Abstract
Adhesion to the intestinal mucosa is one of the main selection criteria for probiotic strains. The adhesion of commonly used probiotic strains to human intestinal tissue pieces and mucus was assessed. The strains tested adhered to the intestinal tissue at low levels and adhered to the intestinal mucus at higher levels.
Lactic acid bacteria (LAB) are commonly used to balance aberrant intestinal microflora and manage microflora dysfunctions (5, 6). Adhesion to the intestinal mucosa is considered important for beneficial health effects (2). In order to assess the adhesive abilities of LAB, in vitro adhesion models have been developed. The most widely used models are tissue culture cells (1, 13) and an intestinal mucus model (8, 9). These models allow the rapid screening of relatively large numbers of bacteria, and although differences in adhesion are sometimes found between these methods, their results are usually in good agreement (10, 13). Recent reports on adhesion and competitive exclusion indicate that these models may not be sufficient to describe the mucosal interactions (4, 9). The present study focused on the development of a model that combines the epithelium, mucus, and the resident mucosal microflora by using resected colonic tissue samples obtained during surgery. The adhesion properties of five well-defined strains of LAB were assessed, and the levels of adhesion of the strains to colonic mucus were compared. In addition, adhesion to different parts of the colon was assessed.
Five strains of LAB were used: Lactobacillus rhamnosus GG (ATCC 53103; Valio Ltd., Helsinki, Finland); Lactobacillus casei Shirota (Yakult Private Ltd., Singapore); Lactobacillus johnsonii La1, isolated from a Nestlé LC1 product; Lactobacillus bulgaricus (ATCC 11842); and Bifidobacterium lactis Bb12 (Chr. Hansen, Hørsholm, Denmark). The bacteria were grown as described earlier (8, 9) and metabolically labeled with tritiated thymidine. The bacterial cultures were washed and resuspended in phosphate-buffered saline (PBS; pH 7.2). The absorbance was adjusted to 0.25 ± 0.02 to standardize the number of bacteria (107 to 108 CFU/ml) before the adhesion assay (see below). The use of resected human intestinal material was approved by the Joint Ethical Committee of the University of Turku and Turku University Central Hospital. Tissue samples were obtained from four patients with colon cancer. Patients 1 to 3 were women (ages, 86, 79, and 55 years, respectively), and patient 4 was a man (age, 45 years). Material was obtained from the descending colons of three patients, and the whole colon was obtained from one patient (patient 4). Five areas of the colon from patient 4 were investigated: cecum, ascending colon, right transverse colon, left transverse colon, and descending colon. The healthy part of the resected tissue was used for the studies, and the patients had no preobstructive lesions. The resected material was collected on ice within 20 min after resection and was processed immediately. The intestine was cut and washed gently (to leave the mucus layer intact) in PBS containing 0.01% gelatin until no contents were visible. Circular pieces of 9 mm were stamped from the tissue. The pieces were stored at −70°C in PBS with 40% glycerol until use. The viabilities of the pieces were not assessed. However, no significant differences in levels of adhesion were observed between fresh and frozen samples (data not shown).
Mucus was collected from part of the washed tissue and placed in a small amount of 10 mM HEPES-Hanks buffer (HH; pH 7.4) by gently scraping the mucosa with a rubber spatula. The collected mucus was centrifuged at 13,000 × g for 10 min to remove the cell debris and bacteria and was stored at −70°C until use.
The adhesion of the radioactively labeled bacteria to immobilized colonic mucus was determined as described previously (8, 9). Adhesion was expressed as the percentage of radioactivity recovered after adhesion relative to the amount of radioactivity in the bacterial suspensions added to the immobilized mucus.
For determination of the level of adhesion to human colonic tissue, a method modified from that of Henriksson and coworkers (3) was used. In short, tissue pieces were thawed at room temperature and gently washed in HH buffer. Radioactively labeled bacteria were allowed to bind for 1 h with incubation at 37°C. After the tissue pieces were washed with PBS they were fixed with glutaraldehyde. The mucosa was then separated and digested overnight with H2O2 and perchloric acid at 60 to 70°C. After digestion, the radioactivity was determined by liquid scintillation counting. The percent adhesion was determined as mentioned above for the colonic mucus (9). The results from the adhesion experiments with mucus and colonic tissue are expressed as the average (standard deviation [SD]) of three independent experiments.
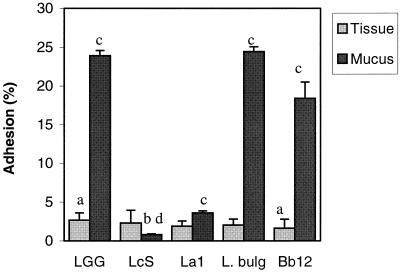
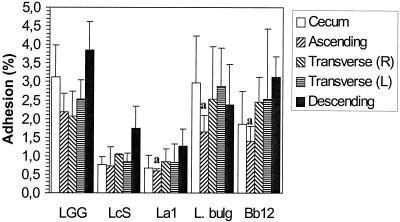
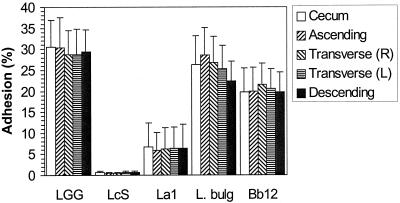
The adhesion to the colonic tissue pieces of the strains tested was found to range from 1.6% (SD, 1.2%) for B. lactis Bb12 to 2.7% (SD, 0.9%) for Lactobacillus GG. Lactobacillus GG was found to adhere significantly better than B. lactis Bb12 (P < 0.05; Wilcoxon signed rank test); no other significant differences in adhesion to human colonic tissue were observed (Fig. 1). The relatively low level of adhesion of lactobacilli to nonlymphoid intestinal tissue is in agreement with earlier in vivo observations (12). The strains tested exhibited a much wider range of colonic mucus adherence abilities, from 0.8% (SD, 0.1%) for L. casei Shirota to 24.5% (SD, 0.6%) for L. bulgaricus. L. casei Shirota was found to adhere significantly less than any of the other strains tested (P < 0.05; Wilcoxon signed rank test). The adhesive abilities of Lactobacillus GG, L. bulgaricus, and B. lactis Bb12 were not different from each other (Fig. 1). The results obtained for adhesion to the mucus were similar to those reported earlier (8, 9). No significant differences in the adhesion of the strains tested were observed between the four subjects (P > 0.05). L. casei Shirota adhered significantly better to colonic tissue than to mucus (P < 0.05; Wilcoxon signed rank test). The other strains tested adhered significantly better to immobilized colonic mucus than to colonic tissue (P < 0.05). Differences in adhesion to the tissue and the corresponding mucus can be explained by the presence of a normal microflora on the former and the absence of bacteria (<100 CFU/ml) from the latter. The resident microflora may affect the adhesion to the colonic tissue of the probiotic strains tested and is usually not taken into account in adhesion models. The competitive exclusion provided by the normal intestinal microflora is generally considered a major function of the normal microflora (7). Also, small food particles and enzymes can interfere with adhesion to colonic tissue but do not interfere with adhesion to mucus (10). The present method offers new ways of characterizing adhesion in relation to the colonic environment. The presence of disease may also have influenced the composition of the mucosal microflora (11), which in turn may affect the adhesion of the probiotic strains; this requires further assessment. The level of adhesion to tissue from the ascending colon was found to be significantly lower (P < 0.05; Friedman test) for L. bulgaricus, L. johnsonii La1, and B. lactis Bb12 than the level of adhesion to tissue from other parts of the colon (Fig. 2). However, the differences are small and it is not clear that they are biologically relevant. No site-specific adhesion to mucus isolated from different parts of the colon was observed (Fig. 3). It is important that the adhesion to other parts of the intestine also be investigated since the intestinal microflora differs quantitatively and qualitatively along the length of the gastrointestinal tract, with there being differences in the microbes that adhere to the intestinal mucus lining and those that directly interact with the epithelial cells (6).
FIG. 1.
Adhesion of five probiotic bacteria to human colonic tissue and isolated human colonic mucus. Results are expressed as the mean of three independent experiments with samples from four individuals. Error bars indicate SDs. a, significantly different adhesion to colonic tissue; b, significantly lower level of adhesion to colonic mucus compared to that for all other strains; c, significantly higher level of adhesion to mucus; d, significantly higher level of adhesion to tissue. LGG, L. rhamnosus GG; LcS, L. casei Shirota; La1, L. johnsonii La1; L. bulg., L. bulgaricus (ATCC 11842); and Bb12, B. lactis Bb12.
FIG. 2.
Adhesion of five probiotic bacteria to tissue from different parts of a human colon (from one individual). Error bars indicate SDs. a, significantly lower levels of adhesion to tissue from descending colon. R, right; L, left; LGG, L. rhamnosus GG; LcS, L. casei Shirota; La1, L. johnsonii La1; L. bulg., L. bulgaricus (ATCC 11842); and Bb12, B. lactis Bb12.
FIG. 3.
Adhesion of five probiotic bacteria to mucus from different parts of a human colon (from one individual). Error bars indicate SDs. R, right; L, left; LGG, L. rhamnosus GG; LcS, L. casei Shirota; La1, L. johnsonii La1; L. bulg., L. bulgaricus (ATCC 11842); and Bb12, B. lactis Bb12.
In conclusion, the results presented here provide a basis for further development of resected intestinal samples as a model for assessment of the adhesion of LAB, taking into account the interactions among the gastrointestinal epithelium, mucus, and the host’s mucosal microflora. Further development of this method offers a means of improving present methods for adhesion in vitro and offers a novel means of, for example, selecting therapeutic LAB and characterizing the interactions with the resident microflora.
Acknowledgments
Financial support for this study was obtained from the Academy of Finland.
REFERENCES
- 1.Coconnier, M.-H., M.-F. Bernet, S. Kernéis, G. Chauvière, J. Fourniat, and A. L. Servin. 1993. Inhibition of adhesion of enteroinvasive pathogens to human intestinal Caco-2 cells by Lactobacillus acidophilus strain LB decreases bacterial invasion. FEMS Microbiol. Lett. 110:299–306. [DOI] [PubMed] [Google Scholar]
- 2.Havenaar, R., B. ten Brink, and J. H. J. Huis in ’t Veld. 1992. Selection of strains for probiotic use, p.209–224. In R. Fuller (ed.), Probiotics: the scientific basis. Chapman & Hall, London, United Kingdom.
- 3.Henriksson, A., R. Szewzyk, and P. L. Conway. 1991. Characteristics of the adhesive determinants of Lactobacillus fermentum 104. Appl. Environ. Microbiol. 57:499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee, Y. K., C. Y. Lim, W. L. Teng, A. C. Ouwehand, E. M. Tuomola, and S. Salminen. 2000. Quantitative approach in the study of adhesion of lactic acid bacteria to intestinal cells and their competition with enterobacteria. Appl. Environ. Microbiol. 66:3692–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattila-Sandholm, T., J. Mättö, and M. Saarela. 1999. Lactic acid bacteria with health claims—interactions and interference with gastrointestinal flora. Int. Dairy J. 9:25–35. [Google Scholar]
- 6.McCracken, V. J., and R. G. Lorenz. 2001. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol. 3:1–11. [DOI] [PubMed] [Google Scholar]
- 7.McFarland, L. V. 2000. Normal flora: diversity and functions. Microb. Ecol. Health Dis. 12:193–207. [Google Scholar]
- 8.Ouwehand, A. C., P. V. Kirjavainen, M.-M. Grönlund, E. Isolauri, and S. J. Salminen. 1999. Adhesion of probiotic microorganisms to intestinal mucus. Int. Dairy J. 9:623–630. [Google Scholar]
- 9.Ouwehand, A. C., E. M. Tuomola, Y. K. Lee, and S. Salminen. 2001. Microbial interactions to intestinal mucosal models. Methods Enzymol. 337:200–212. [DOI] [PubMed] [Google Scholar]
- 10.Ouwehand, A. C., S. Tölkkö, and S. Salminen. 2001. The effect of digestive enzymes on the adhesion of probiotic pacteria in vitro. J. Food Sci. 66:856–859. [Google Scholar]
- 11.Pathmakanthan, S., J. P. Thornley, and C. J. Hawkey. 1999. Mucosally associated bacterial flora of the human colon: quantitative and species specific differences between normal and inflamed colonic biopsies. Microb. Ecol. Health Dis. 11:169–174. [Google Scholar]
- 12.Plant, L., and P. Conway. 2001. Association of Lactobacillus spp. with Peyer’s patches in mice. Clin. Diagn. Lab. Immunol. 8:320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuomola, E. M., and S. J. Salminen. 1998. Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int. J. Food Microbiol. 41:45–51. [DOI] [PubMed] [Google Scholar]