Abstract
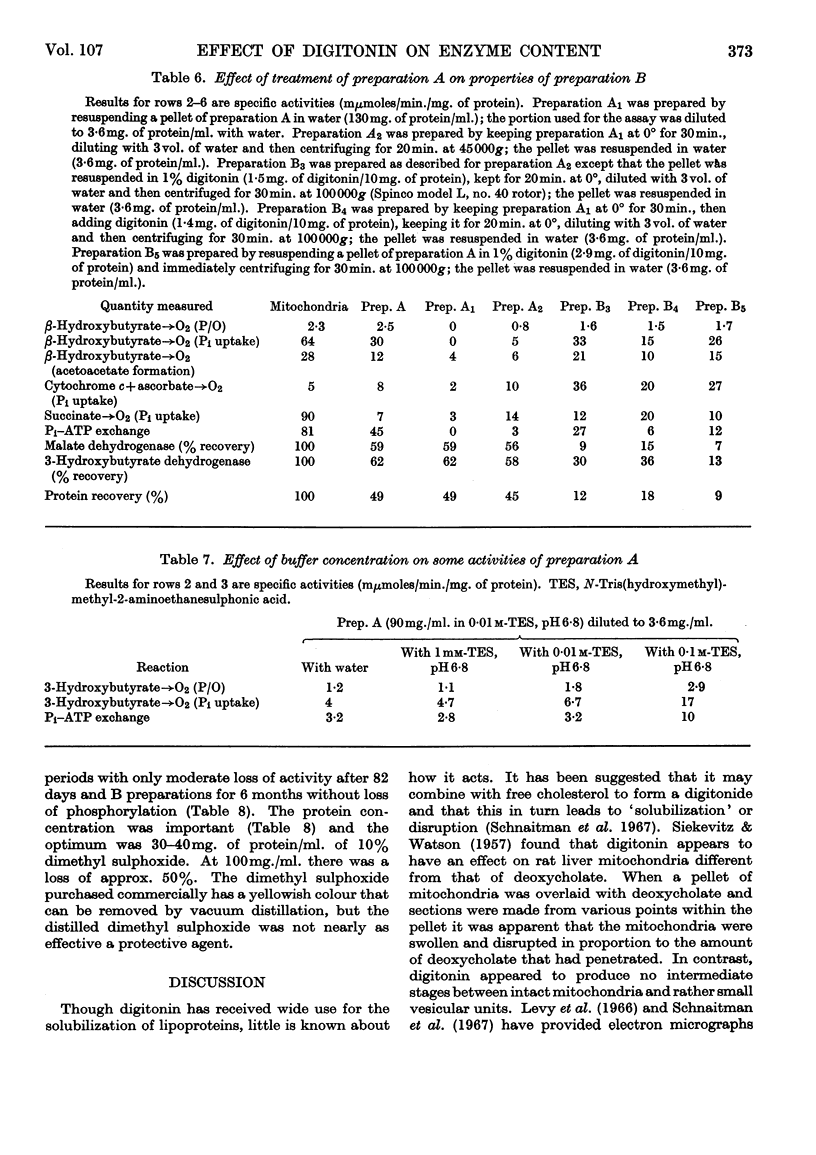
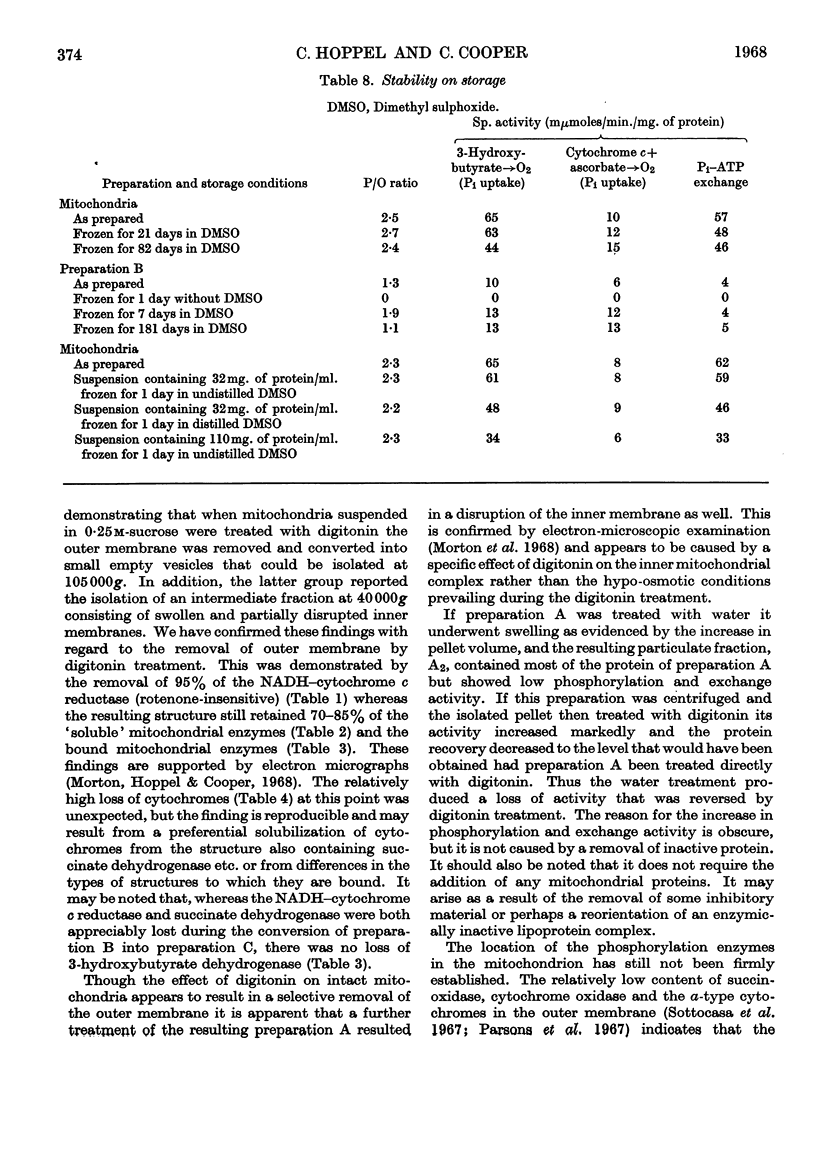
1. The effects of repetitive treatment of rat liver mitochondria with digitonin were examined. The first treatment results in the removal of the outer membrane. Almost all the NADH–cytochrome c reductase (rotenone-insensitive) is lost whereas the major portions of the soluble and bound enzymes are retained. One exception appears to be the cytochromes, which undergo somewhat larger losses. The resulting inner-membrane complex carries out oxidative phosphorylation and Pi–ATP exchange. 2. The properties of the inner-membrane complex are affected by the osmoticity of the medium. When it is suspended in water little protein is lost but there is a marked loss of phosphorylation. If after the suspension in water the particulate fraction is reisolated by centrifugation and treated with digitonin, or if the aqueous suspension is treated directly with digitonin and the particulate fraction then reisolated, the phosphorylation is largely restored. 3. Additional treatment of the inner mitochondrial complex with digitonin results in the formation of a particulate fraction that contains approx. 8% of the initial mitochondrial protein, no outer membrane, no soluble mitochondrial enzymes and is still capable of coupled oxidative phosphorylation and Pi–ATP exchange. These effects cannot be reproduced by treatment with water. 4. The rat liver mitochondria and all of the resulting preparations obtained after digitonin treatment may be stored for long periods in dimethyl sulphoxide with little change of activity.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- COOPER C., KULKA R. G. Properties of the inorganic orthophosphate-adenosine triphosphate and adenosine diphosphate-adenosine triphosphate exchange reactions of digitonin particles. J Biol Chem. 1961 Aug;236:2351–2356. [PubMed] [Google Scholar]
- COOPER C., LEHNINGER A. L. Oxidative phosphorylation by an enzyme complex from extracts of mitochondria. I. The span beta-hydroxybutyrate to oxygen. J Biol Chem. 1956 Mar;219(1):489–506. [PubMed] [Google Scholar]
- Caplan A. I., Greenawalt J. W. Biochemical and ultrastructural properties of osmotically lysed rat-liver mitochondria. J Cell Biol. 1966 Dec;31(3):455–472. doi: 10.1083/jcb.31.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREIFF D., MYERS M. Effect of dimethyl sulphoxide on the cryo-tolerance of mitochondria. Nature. 1961 Jun 24;190:1202–1204. doi: 10.1038/1901202b0. [DOI] [PubMed] [Google Scholar]
- JURTSHUK P., Jr, SEKUZU I., GREEN D. E. STUDIES ON THE ELECTRON TRANSFER SYSTEM. LVI. ON THE FORMATION OF AN ACTIVE COMPLEX BETWEEN THE APO-D(--)-BETA-HYDROXYBUTYRIC DEHYDROGENASE AND MICELLAR LECITHIN. J Biol Chem. 1963 Nov;238:3595–3605. [PubMed] [Google Scholar]
- KULKA R. G., COOPER C. The action of oligomycin on the inorganic orthophosphate-adenosine triphosphate and adenosine diphosphate-adenosine triphosphate exchange reactions of digitonin particles. J Biol Chem. 1962 Mar;237:936–939. [PubMed] [Google Scholar]
- Lévy M., Toury R., André J. Essai de séparation des deux membranes mitochondriales. C R Acad Sci Hebd Seances Acad Sci D. 1966 Apr 4;262(14):1593–1596. [PubMed] [Google Scholar]
- Morton D. J., Hoppel C., Cooper C. The action of digitonin on rat liver mitochondria. Electron microscopy. Biochem J. 1968 Apr;107(3):377–380. doi: 10.1042/bj1070377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- SIEKEVITZ P., WATSON M. L. Some cytochemical characteristics of a phosphorylating digitonin preparation of mitochondria. Biochim Biophys Acta. 1957 Aug;25(2):274–279. doi: 10.1016/0006-3002(57)90469-9. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADKINS C. L., LEHNINGER A. L. The adenosine triphosphate-adenosine diphosphate exchange reaction of oxidative phosphorylation. J Biol Chem. 1958 Dec;233(6):1589–1597. [PubMed] [Google Scholar]
- WALKER P. G. A colorimetric method for the estimation of acetoacetate. Biochem J. 1954 Dec;58(4):699–704. doi: 10.1042/bj0580699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTERS E., COOPER C. POSSIBLE SOURCES OF ERROR IN THE DETERMINATION OF ORGANIC PHOSPHATE BY THE ISOBUTANOL-BENZENE EXTRACTION METHOD. Anal Biochem. 1965 Feb;10:370–375. doi: 10.1016/0003-2697(65)90282-4. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J. N., Jr A METHOD FOR THE SIMULTANEOUS QUANTITATIVE ESTIMATION OF CYTOCHROMES A, B, C1, AND C IN MITOCHONDRIA. Arch Biochem Biophys. 1964 Sep;107:537–543. doi: 10.1016/0003-9861(64)90313-3. [DOI] [PubMed] [Google Scholar]


