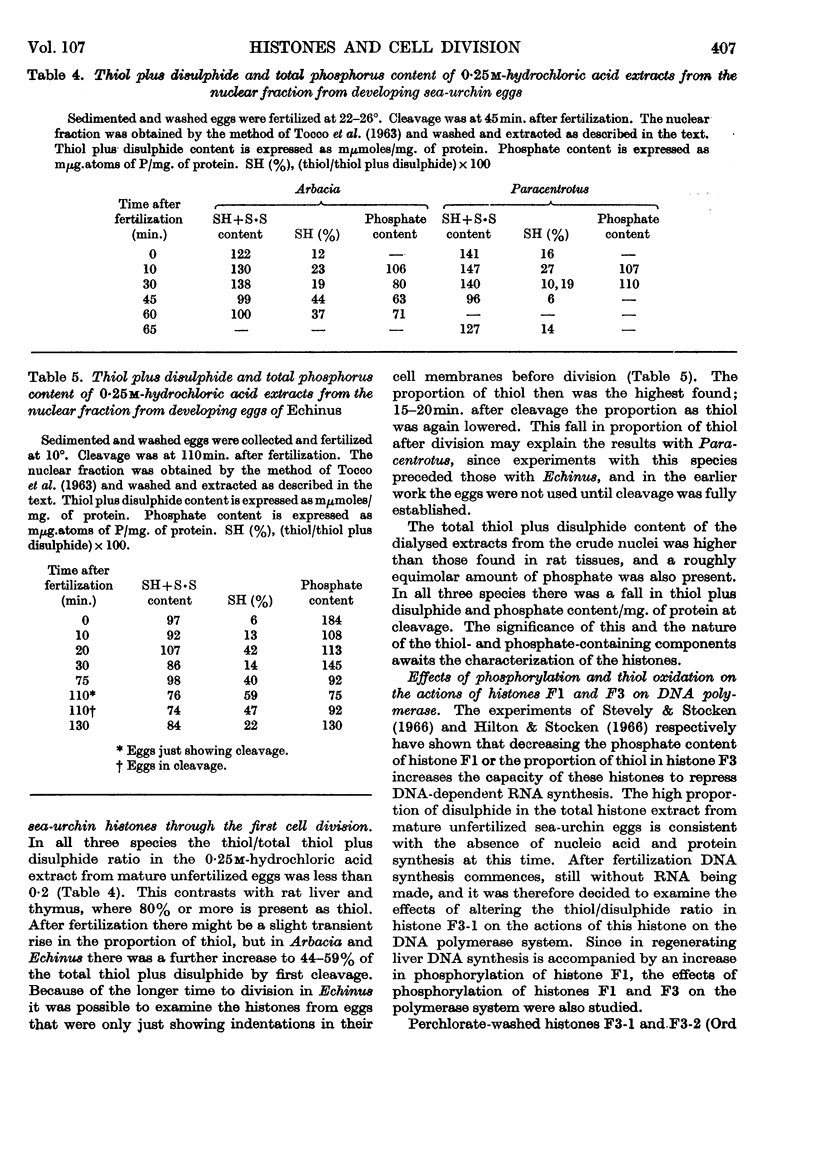
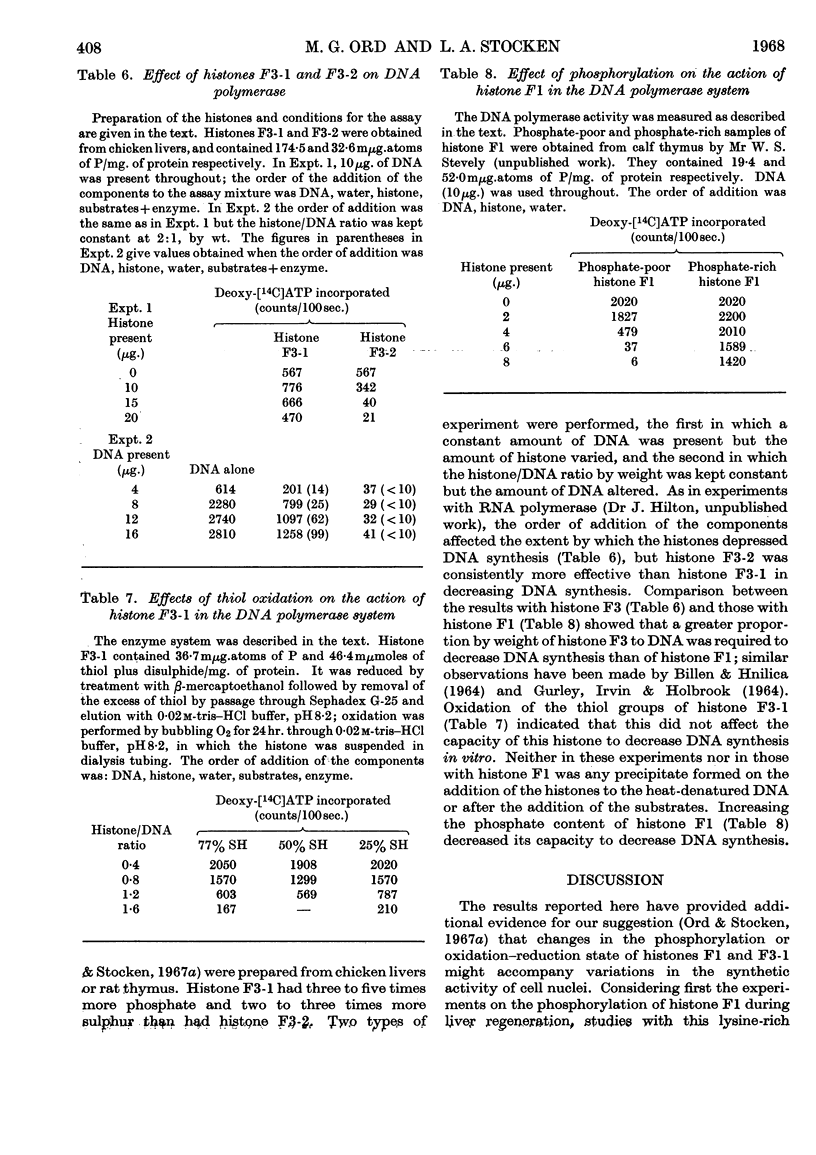
Abstract
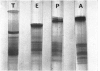
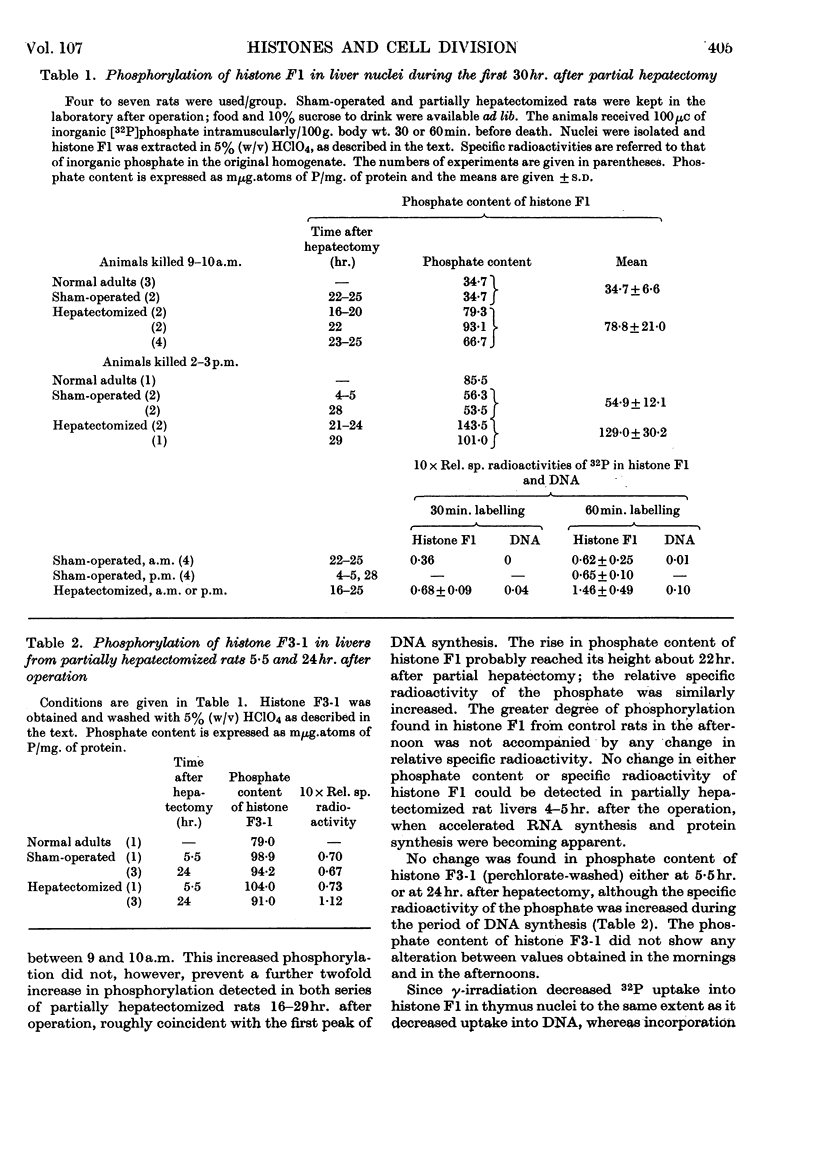
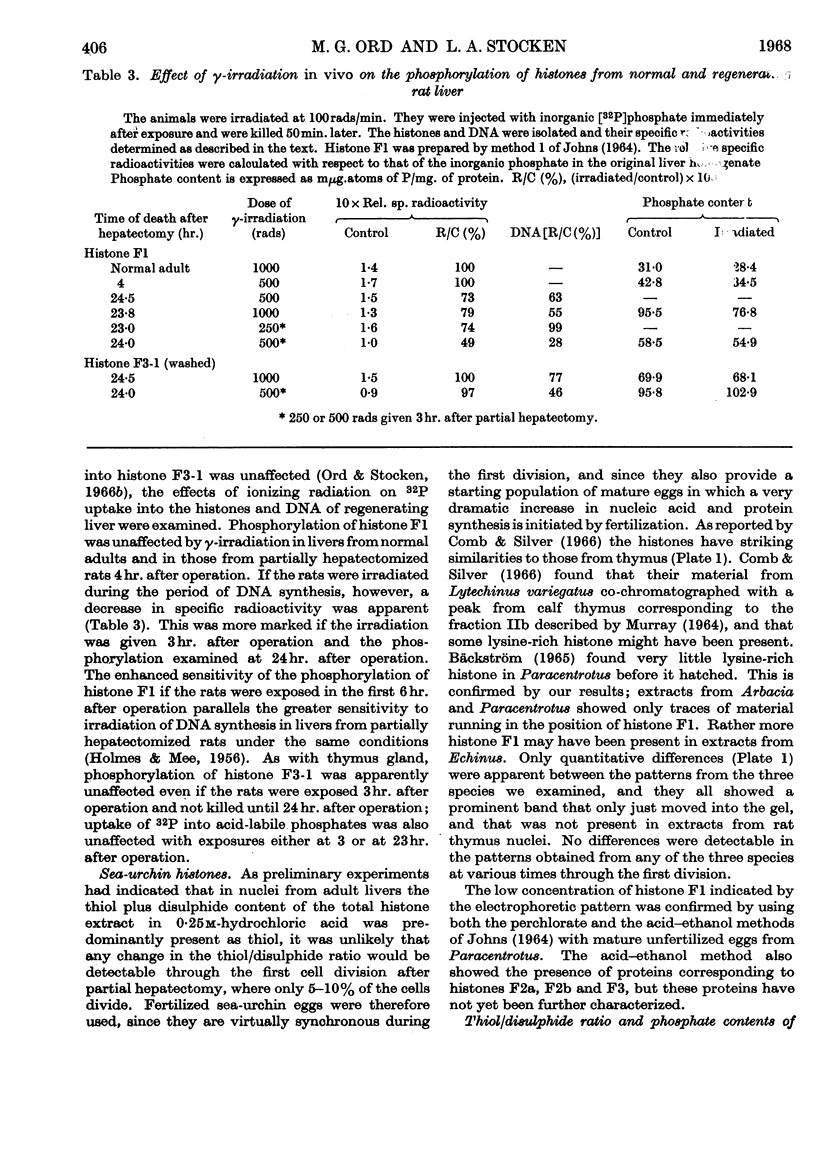
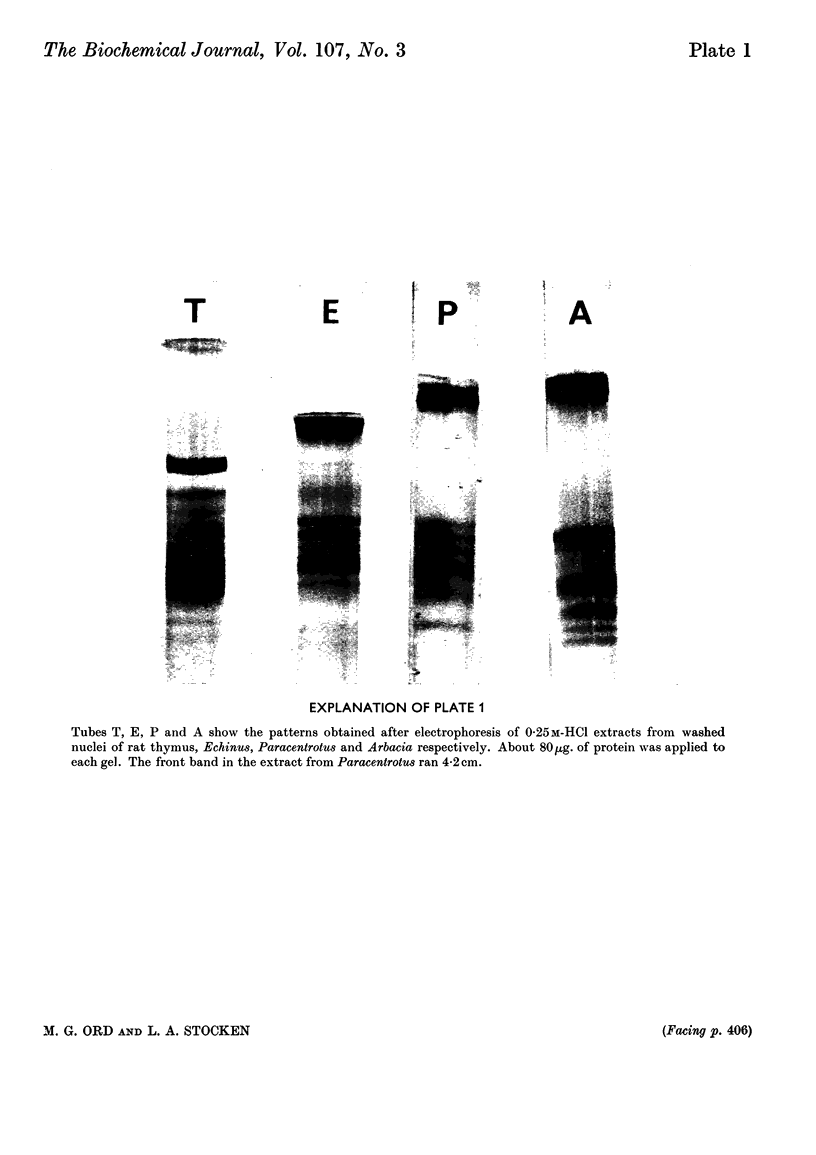
1. In regenerating rat liver the phosphate content of the lysine-rich histone F1, but not that of the more arginine-rich histone F3-1, increases during the period of DNA synthesis. 2. The phosphorylation of histone F1 in this `S period' is decreased by γ-irradiation, but, like phosphate uptake into DNA, is affected to an even greater extent if the irradiation is given in the presynthetic period. 3. Histones from three species of sea-urchin eggs show similarities to the F2 and F3 groups of histones from mammalian thymus gland. 4. The proportion of thiol to total thiol plus disulphide in acid extracts from sea-urchin eggs varies from less than 20% in mature unfertilized eggs to 59% just before cleavage. 5. The phosphorylated forms of histones F1 and F3 are less effective in decreasing DNA synthesis by DNA polymerase than the non-phosphorylated forms. 6. Oxidation of thiol groups on histone F3-1 does not affect its capacity to decrease DNA synthesis in vitro.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BOLLUM F. J. Calf thymus polymerase. J Biol Chem. 1960 Aug;235:2399–2403. [PubMed] [Google Scholar]
- Berenblum I., Chain E. An improved method for the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckström S. Basic proteins in the sea urchin embryo (Paracentrotus lividus). Acta Embryol Morphol Exp. 1965 May;8(1):20–31. [PubMed] [Google Scholar]
- Comb D. C., Silver D. J. Synthesis of basic proteins and cellular RNA species during sea urchin development. Natl Cancer Inst Monogr. 1966 Dec;23:325–336. [PubMed] [Google Scholar]
- DELUCA H. A., ROSSITER R. J., STRICKLAND K. P. Incorporation of radioactive phosphate into the nucleic acids of brain slices. Biochem J. 1953 Sep;55(2):193–200. doi: 10.1042/bj0550193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Frenster J. H. Nuclear polyanions as de-repressors of synthesis of ribonucleic acid. Nature. 1965 May 15;206(985):680–683. doi: 10.1038/206680a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. On the origin and persistence of a cytoplasmic state inducing nuclear DNA synthesis in frogs' eggs. Proc Natl Acad Sci U S A. 1967 Aug;58(2):545–552. doi: 10.1073/pnas.58.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley L. R., Irvin J. L., Holbrook D. J. Inhibition of DNA polymerase by histones. Biochem Biophys Res Commun. 1964;14:527–532. doi: 10.1016/0006-291x(64)90263-3. [DOI] [PubMed] [Google Scholar]
- Ingles C. J., Dixon G. H. Phosphorylation of protamine during spermatogenesis in trout testis. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1011–1018. doi: 10.1073/pnas.58.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAFFE J. J. Diurnal mitotic periodicity in regenerating rat liver. Anat Rec. 1954 Dec;120(4):935–954. doi: 10.1002/ar.1091200408. [DOI] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marsh W. H., Ord M. G., Stocken L. A. Thiol proteins in nuclei from rat liver and thymus. Biochem J. 1964 Dec;93(3):539–544. doi: 10.1042/bj0930539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauritzen C. M., Starbuck W. C., Saroja I. S., Taylor C. W., Busch H. The fractionation of arginine-rich histones from fetal calf thymus by exclusion chromatography. J Biol Chem. 1967 May 10;242(9):2240–2245. [PubMed] [Google Scholar]
- ORD M. G., RAAF J. H., SMIT J. A., STOCKEN L. A. METABOLIC AND CHEMICAL PROPERTIES OF BASIC PROTEINS ISOLATED FROM NUCLEI OF RAT LIVER AND THYMUS GLAND. Biochem J. 1965 May;95:321–331. doi: 10.1042/bj0950321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A. Metabolic properties of histones from rat liver and thymus gland. Biochem J. 1966 Mar;98(3):888–897. doi: 10.1042/bj0980888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A. Phosphate and thiol groups in histone f3 from rat liver and thymus nuclei. Biochem J. 1967 Feb;102(2):631–636. doi: 10.1042/bj1020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter V. R., Gebert R. A., Pitot H. C. Enzyme levels in rats adapted to 36-hour fasting. Adv Enzyme Regul. 1966;4:247–265. doi: 10.1016/0065-2571(66)90020-3. [DOI] [PubMed] [Google Scholar]
- Sakai H. A ribonucleoprotein which catalyzes thiol-disulfide exchange in the sea urchin egg. J Biol Chem. 1967 Apr 10;242(7):1458–1461. [PubMed] [Google Scholar]