ABSTRACT
Background
Nitrate reductases (NR) expressed in oral bacteria reduce nitrate to nitrite. Depending on the environmental conditions and types of bacteria present nitrite can be further reduced to ammonium via Dissimilatory Nitrate Reduction to Ammonium (DNRA), or alternatively to nitric oxide (NO), which impacts cardiometabolic health.
Objective
To evaluate the associations between nitrate reduction by salivary bacteria, clinical markers of glucose metabolism, and lifestyle factors that can modulate the oral environment, potentially impacting DNRA and NR expression.
Methods
A cross-sectional study was conducted using a convenience sample of 144 participants from the San Juan Overweight Adult Longitudinal Study (SOALS), which includes data on glucose metabolism and lifestyle. DNRA and NR activities were measured in saliva under aerobic or CO2-enriched conditions.
Results
DNRA activity was inversely associated with insulin resistance (HOMA-IR) [aerobic3rd vs.1st tertile: β=-0.48 (−0.81, −0.15); CO2-enriched3rd vs.1st tertile β=-0.42 (−0.68, −0.17)], fasting blood glucose [aerobic3rd vs.1st tertile β=-0.144 (−0.268, −0.019); CO2-enriched3rd vs.1st tertile: β=-0.070 (−0.130, −0.011)], and 2-h glucose [CO2-enriched3rd vs.1st tertileβ=-0.21 (−0.37, −0.04)]. Current smokers had lower DNRA activity than non-smokers under aerobic conditions [β=-1.55 (−2.96, −0.14)], but higher under CO2-enriched conditions [β = 0.93 (0.15, 1.71)]. Toothbrushing frequency (twice/day vs. once/day) was positively associated with DNRA activity under CO2-enriched conditions [β = 4.11 (1.90, 6.32)] and with aerobic NR activity [β = 1.20, (0.14, 2.27)]. Physical activity was inversely associated with aerobic NR [β=-0.01, (−0.022, −0.003)]. Under CO2-enriched conditions NR was inversely associated with the BMI (β=-0.11, p = 0.007). Aerobic NR was higher when sucrose was added to the assays (NADP vs. sucrose β=-0.74, p = 0.02) and positively associated with salivary nitrate levels (β = 0.002, p = 0.002).
Conclusions
Nitrate reduction by salivary bacteria is inversely associated with insulin resistance and can be modulated by lifestyle factors. This knowledge could lead to the development of novel, non-invasive approaches for monitoring and preventing diabetes progression.
KEYWORDS: Nitric oxide, nitrate, nitrite, ammonium, insulin resistance, salivary microbiome, brushing, smoking, diabetes, lifestyle
ONE SENTENCE SUMMARY
This study provides new evidence regarding the role of the salivary microbiome in glucose metabolism, which could be applied to the development of novel, saliva-based, non-invasive approaches for monitoring and preventing diabetes progression.
Introduction
The reduction of nitrate (NO3−) by nitrate reductases encoded in oral bacteria is a critical step in the generation of nitric oxide (NO) through the entero-salivary pathway [1]. NO is an endogenous signaling molecule that plays an important homeostatic role in endothelial function and glucose metabolism [2,3]. The oral microbiome’s role in NO metabolism was established via its effect on blood pressure regulation, but its contribution to diabetes through nitrate reduction is relatively less understood.
A higher abundance of nitrate-reducing bacteria in the subgingival dental plaque has been associated with reduced insulin resistance [4], while other nitrate-reducing bacteria, such as Prevotella and Veillonella, have been associated with impaired glucose metabolism [5]. Oral nitrate supplementation improves insulin sensitivity in obese individuals, and this effect is abolished by antibacterial mouthwash [6]. Our longitudinal study on >1,000 overweight adults in Puerto Rico (San Juan Overweight Adult Longitudinal Study – SOALS) found the use of over-the-counter (OTC) mouthwash twice a day or more was associated with a 55% higher risk of developing pre-diabetes or diabetes during a three-year observation period, after adjusting for confounders [7]. Collectively, these clinical observations suggest that oral bacteria may impact glucose metabolism through nitrate reduction, but direct evidence regarding this association in humans is lacking.
The reduction of nitrate (NO3−) by bacterial nitrate reductase (NR) initially generates nitrite (NO2−), which is then further reduced either to ammonium (NH4+) via dissimilatory (DNRA-NrfA) or assimilatory (NirB, AniA) pathways, or to NO via denitrification (NirK, NirS). In natural ecosystems, these pathways are controlled by environmental parameters, including oxygen availability and type of electron donors vs. electron acceptors available, for example, carbon-to-nitrate ratio and nitrate-to-nitrite ratio [8–10]. In the oral environment, these parameters could be modulated by lifestyle factors, such as oral hygiene practices, smoking, and diet [11]. Therefore, the objectives of this study were to evaluate associations between nitrate reduction pathways in salivary bacteria and clinical markers of glucose metabolism, and to assess the impact of lifestyle and other host factors on these pathways.
Methods
Participants
A cross-sectional study was conducted using baseline saliva samples and data from a subset of participants in the SOALS [7]. The study was approved by the University of Puerto Rico Human Research Subjects Protection Office Institutional Review Board (IRB #2290032918) and is reported following STROBE guidelines. The recruitment of the participants and sample collection took place between 2011 and 2013; saliva and blood samples were stored at −80°C under controlled conditions. SOALS cohort participants were overweight adults aged between 40 and 65 years without physician-diagnosed type 1 or type 2 diabetes who were not taking insulin or oral anti-hyperglycemic medications [12,13]. Exclusionary criteria for the SOALS cohort included pregnancy, hypoglycemia, congenital heart murmurs or heart disease, heart valve disease, endocarditis, rheumatic fever, bleeding disorders, and active dialysis treatment. The present study included a convenience sub-sample of 144 baseline saliva samples (pellets and supernatants stored separately) from consecutively recruited SOALS participants with the following additional eligibility criteria applied: a) participants were not taking antibiotics, and b) a sufficient amount of saliva sample was available for the proposed assays. Clinical, behavioral, and sociodemographic metadata were available from SOALS with collection methods described below.
Clinical procedures and sample collection
Clinical procedures for the collection of blood and saliva samples in SOALS have been previously described in detail [7]. Briefly, participants were asked to fast for 10 h before the study visit. Blood samples were drawn during fasting at 30 min, 1-h, and 2-h after consuming a glucose drink containing 75 g dextrose, using a standard protocol and silicone-coated sterile blood collection tubes (Becton Dickinson Vacutainer Systems, NJ). Blood was centrifuged to separate red blood cells (RBCs) and serum/plasma, and EDTA tubes for plasma samples and serum were stored at −80°C. Fasting blood was processed for glucose, insulin, and HbA1c. Glucose was assessed using a SIRRUS analyzer (intra-assay coefficient of variation 1.21%; inter-assay 3.06%). Insulin was measured using chemiluminescence assays with a TOSOH analyzer (intra-assay coefficient of variation 1.49%; inter-assay 4.42%). Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) was calculated as (Fasting glucose × Fasting insulin)/405. HbA1c was assessed using a latex immunoagglutination inhibition method (intra-assay coefficient of variation 2.89% and inter-assay 1.88%). Whole unstimulated saliva was collected 30 min after administering the glucose drink. Saliva samples were centrifuged 2600 × g for 15 min at 4°C; pellets containing the salivary bacteria were stored separately from the saliva supernatant at −80°C.
In the SOALS cohort, interviewer-administered questionnaires were used to assess the frequency of oral hygiene practices, including mouthwash use. The questionnaire collected information on important covariates, including age, gender, smoking, alcohol intake, and time and frequency of physical activity during a typical week. Each activity was assigned a metabolic equivalent (MET) score based on the intensity, and the total MET score was computed for each individual as MET hours/week. Anthropometric measurements were taken according to the NHANES III procedures, and replicate measures were averaged.
Nitrate reduction assays
Nitrate reduction assays were performed between 2021 and 2022 using salivary pellets. Salivary pellets were defrosted on ice, resuspended in PBS and incubated with 50 mm potassium nitrate for 2–3 h at 37°C under aerobic conditions (ambient air) or CO2-enriched conditions (BD GasPakTM EZ CO2, New Jersey, USA), which are more similar to the oral environment and exhaled air [14,15]. The reactions included 1 mm of either NADP or sucrose, which have been shown to affect nitrate reduction in our pilot experiments [16]. Control reactions without nitrate were included for each sample. Nitrite was measured using the SieversTM Nitric Oxide Analyzer NOA 280i instrument (Zysense, NC), and ammonium was measured with the Nessler’s reagent using ammonium sulfate as standard (Fisher Scientific, Fair Lawn, NJ, USA). Protein concentration of the salivary suspensions was determined with the Bradford method using bovine serum albumin as standard (Bio Rad, Hercules, CA, USA). DNRA activity was defined as nmoles NH4+/min/mg of protein, and NR activity as nmoles NO2−/min/mg of protein.
Nitrate and nitrite measurements in saliva samples
Nitrate and nitrite were measured in saliva supernatants, stored separately from the pellets. After thawing, saliva was centrifuged (5000 × g, 10 min), and supernatant nitrite and nitrate were measured by HPLC-coupled to the Griess reaction (Eicom). Nitrite levels were also measured by triiodide reduction to NO and measurement by reaction with ozone using the Sievers NO-analyzer [17].
Statistical procedures
Descriptive analyses were presented and compared to overall SOALS participants (Table 1). Continuous variables (HOMA-IR, HbA1c, fasting glucose, 2-h glucose, aerobic and CO2-enriched DNRA, and NR) were all larger than zero, positively skewed, and widely distributed. Therefore, measurements in the descriptive table were expressed as median and interquartile ranges (IQR); other continuous measurements used mean and standard deviation (SD), and count data were used percentages. One outlier in the CO2-enriched DNRA variable (55 nmoles NH4+/min/mg) was replaced with a missing value to ensure data integrity. In the primary analysis, we assessed the association between DNRA/NR levels as exposures (using tertiles) and glucose metabolism-related outcomes (HOMA-IR, HbA1c, fasting glucose, 2-h glucose) . Gamma regression models were employed because these continuous outcomes were severely positively skewed, and conventional linear regression models, even when using their transformed levels as outcomes, were no longer appropriate [7]. Gamma regression is widely recommended for modelling continuous outcomes with positive skewness, and it also accommodates heteroscedasticity (non-constant variance) often present in skewed data. This makes Gamma regression a more natural fit and also more robust and reliable for our dataset. Associations were adjusted for age and sex in the simple models and then for covariates such as smoking, BMI, and physical activity in the comprehensive models.
Table 1.
Descriptive statistics of study variables in the entire SOALS vs. the participants of this study: mean (SD) (if not specified) or %.
| SOALS (N = 1342) | Current study (N = 144) | |
|---|---|---|
| Assessments from SOALS baseline | ||
| Age in years | 50.51 (6.72) | 49.76 (6.14) |
| Sex (Female) | 71.54% | 63.19% |
| Smoke status | ||
| None | 62.78% | 54.55% |
| Former | 17.71% | 29.37% |
| Current | 19.51% | 16.08% |
| BMI (kg/m2) | 33.45 (6.33) | 34.70 (6.81) |
| Physical activity: METs | 20.98 (39.00) | 23.39 (29.75) |
| HOMA_IR (mg/dL): median (IQR) | 2.22 (2.11) | 2.20 (2.23) |
| HbA1c (%): median (IQR) | 5.70 (0.50) | 5.80 (0.40) |
| Fasting glucose (mg/dL): median (IQR) | 93.00 (14.00) | 92.00 (13.50) |
| 2-hour glucose (mg/dL): median (IQR) | 113.00 (41.00) | 108.00 (35.00) |
| Mouthwash Use | ||
| <Once a day or never | 55.97% | 51.75% |
| Once a day | 20.97% | 27.97% |
| ≥ twice a day | 23.06% | 20.28% |
| Flossing | ||
| Less than once per week or never | 38.36% | 39.86% |
| Less than once per day | 20.45% | 25.17% |
| Once per day | 21.87% | 21.68% |
| Twice per day or more | 19.33% | 13.29% |
| Brushing | ||
| Less than once per week or no use | 0.07% | 0.00% |
| Once per day | 9.18% | 6.99% |
| Twice per day | 57.69% | 63.64% |
| More than twice per day | 33.06% | 29.37% |
| Laboratory measurements | ||
| Saliva nitrite (µM): median (IQR) | 47.27 (44.56) | 59.72 (58.89) |
| Saliva nitrate (µM): median (IQR) | 99.53 (129.43) | 106.08 (165.21) |
| Electron donor | ||
| Sucrose | —- | 27.78% |
| NADP | —- | 72.22% |
| DNRA_aerobica (N = 65): median (IQR) | —- | 0.11 (3.54) |
| DNRA_CO2b (N = 60): median (IQR) | —- | 1.23 (4.89) |
| NR_aerobica (N = 83): median (IQR) | —- | 0.05 (0.09) |
| NR_CO2b (N = 61): median (IQR) | —- | 0.08 (0.14) |
anmoles NH4+/min/mg protein;
bnmoles NO2-/min/mg protein.
For the same reasons mentioned above, we used Gamma regression models to investigate the association between continuous measurements of DNRA and NR as outcomes and lifestyle factors, such as flossing, smoking, brushing, and mouthwash use. Covariates of age, sex, presence of sucrose vs. NADP in the assay, and BMI were included in the base model, and lifestyle factors were added to the base model one at a time. The coefficients and 95% confidence intervals (CIs) obtained from Gamma regression models were included in the output tables. Analyses were conducted using Stata/SE 16.1, and p < 0.05 was considered statistically significant.
Results
The participants (N = 144, 36.81% males, 63.19% females) were between 40 and 65 years old (mean 49.76 years, SD = 6.14 years) and were all overweight (BMI 25–55, mean = 34.70, SD = 6.81). The sociodemographic and clinical profile of this study’s cohort and salivary nitrate and nitrite levels were similar to the larger SOALS cohort, as shown in Table 1.
Impact of host-related and lifestyle factors on DNRA activity
DNRA and NR were higher in CO2-enriched vs. aerobic conditions (Table 1), but the differences were not statistically significant (NR p = 0.334; DNRA p = 0.097). Under aerobic conditions, NR was lower when NADP was added in the assay instead of sucrose (base model β=-0.74, p = 0.02) but positively associated with salivary nitrate levels (β = 0.002, 95% CI: 0.0007, 0.003). Under CO2-enriched conditions, NR was inversely associated with BMI (base model β=-0.11, p = 0.007). Table 2 shows the significant coefficients for sucrose and BMI in the individual models of each lifestyle factor. Age and sex were not significantly associated with DNRA or NR except in the model for brushing.
Table 2.
Impact of oral hygiene practices, smoking, and other factors on salivary DNRA and nitrate reductase activity.
| Categories |
MODELS: β (95% CI) |
||||
|---|---|---|---|---|---|
| DNRA_ae | DNRA_Co2 | NR_ae | NR_Co2 | ||
| Flossing | ref: <1/week | ||||
| <1/day | 0.15 (−0.76, 1.07) | −0.46 (−1.31, 0.38) | −0.09 (−0.86, 0.68) | −0.13 (−1.20, 0.94) | |
| 1/day | 0.03 (−1.06, 1.13) | −0.48 (−1.51, 0.54) | −0.40 (−1.22, 0.43) | −0.45 (−1.20, 0.30) | |
| >1/day | 0.37 (−0.83, 1.57) | −0.42 (−1.89, 1.05) | −0.67 (−1.37, 0.03) | 0.35 (−0.84, 1.54) | |
| Other Significant Co-variants (95% Cl) | N/A | N/A | NADP vs. sucrose -0.97 (−1.68, −0.26)** |
N/A | |
| Smoking | ref: <1/week | ||||
| former | -1.11 (−2.20, −0.02)* | 0.22 (−0.58, 1.01) | 0.28 (−0.38, 0.93) | 0.04 (−0.85, 0.93) | |
| current | -1.55 (−2.96, −0.14)* | 0.93 (0.15, 1.71)* | −0.21 (−0.95, 0.52) | 0.88 (−0.05, 1.81) | |
| Other Significant Co-variants (95% Cl) | N/A | N/A | NADP vs. sucrose -0.75 (−1.35, −0.15)* |
BMI: -0.10 (−0.18, −0.02)* |
|
| Mouthwash | ref: <1/day | ||||
| 1/day | −0.16 (−1.26, 0.94) | −0.04 (−0.85, 0.76) | −0.0003 (−0.79, 0.79) | −0.04 (−0.82, 0.74) | |
| >1/day | −0.27 (−1.44, 0.90) | 0.61 (−0.38, 1.60) | −0.001 (−0.78, 0.78) | 0.38 (−0.36, 1.12) | |
| Other Significant Co-variants (95% Cl) | N/A | N/A | NADP vs. sucrose -0.73 (−1.38, −0.08)* |
BMI: -0.10 (−0.18, −0.03)** |
|
| Brushing | ref: 1/day | ||||
| 2/day | −0.74 (−2.27, 0.78) | 4.11 (1.90, 6.32)*** | 1.20 (0.14, 2.27)* | 0.83 (−0.37, 2.02) | |
| >2/day | −0.14 (−1.49, 1.21) | 3.06 (0.94, 5.18)** | 0.61 (−0.51, 1.72) | 1.25 (−0.11, 2.60) | |
| Other Significant Co-variants (95% Cl) | N/A | Age: -0.08 (−0.16, −0.008)* Sex: -0.95 (−1.80, −0.10)* |
BMI: -0.05 (−0.10, −0.0004)* |
BMI: -0.11 (−0.19, −0.03)** |
|
| Physical activity | 0.002 (−0.014, 0.018) | 0.005 (−0.006, 0.015) | -0.01 (−0.022, −0.003)** | −0.001 (−0.007, 0.005) | |
| Other Significant Co-variants (95% Cl) | N/A | N/A | NADP vs. sucrose -1.01 (−1.67, −0.34)** |
BMI: -0.12 (−0.19, −0.05)*** |
|
| Saliva nitrite | 0.003 (−0.007, 0.013) | −0.001 (−0.005, 0.002) | 0.004 (−0.0004, 0.008) | −0.006 (−0.015, 0.002) | |
| Other Significant Co-variants (95% Cl) | N/A | N/A | BMI: -0.05 (−0.097, −0.003)* NADP vs. sucrose -0.82 (−1.50, −0.15)* |
N/A | |
| Saliva nitrate | −0.002 (−0.007, 0.002) | −0.001 (−0.002, 0.0003) | 0.002 (0.0007, 0.003)** | −0.003 (−0.008, 0.002) | |
| Other Significant Co-variants (95% Cl) | N/A | N/A | BMI: -0.052 (−0.097, −0.006)* |
BMI: -0.11 (−0.20, −0.02)* |
|
DNRA: nmoles NH4+/min/mg; NR: nmoles NO2−/min/mg; ae: aerobic incubation, Co2: incubation in CO2-enriched (reduced oxygen) conditions. Continuous outcomes were used in Gamma regression models. All models have covariates: age, sex (female vs. male), electron donor (NADP vs. sucrose), and BMI. Additionally, each model (except the base model) includes one ‘lifestyle’ factor (flossing, smoking, mouthwash, and brushing). p-values: *<0.05; **<0.01; ***<0.001.
Current and former smokers had lower DNRA activity than never-smokers under aerobic conditions [current vs. never β=-1.55 (−2.96, −0.14); former vs. never β=-1.11 (−2.20, −0.02)] but higher DNRA activity than never-smokers under CO2-enriched conditions [β = 0.93 (0.15, 1.71)] (Table 2). Frequency of brushing was positively associated with DNRA under CO2-enriched conditions [twice/day vs. once/day β = 4.11 (1.90, 6.32); more than twice/day vs. once/day β = 3.06 (0.94, 5.18)] and with aerobic NR [twice/day vs. once/day β = 1.2, (0.14, 2.27)]. Mouthwash use was not associated with DNRA or NR under any conditions. Physical activity was inversely associated with aerobic NR [β=-0.01, (−0.022, −0.003)].
Association of nitrate reductase and DNRA with glucose metabolism
In multivariate models adjusted for age, sex, BMI, smoking, and physical activity, HOMA-IR levels were negatively associated with DNRA under both aerobic [3rd vs. 1st tertile β= −0.48 (−0.81, −0.15)] and CO2-enriched [3rd vs. 1st tertiles β=-0.42 (−0.68, −0.17)] conditions (Table 3). Under CO2-enriched conditions, DNRA was inversely associated with 2-h glucose levels [β=-0.21 (−0.37, −0.04)], and with fasting glucose in age and sex-adjusted models [2nd vs. 1st tertile: β= −0.064 (−0.127, −0.0007); 3rd vs. 1st tertile β=-0.070 (−0.130, −0.011)]. Under aerobic conditions, DNRA was inversely associated with fasting glucose [β=-0.144 (−0.268, −0.019)] and with HbA1c [β=-0.054 (−0.105, -0.003)]. NR was not statistically associated with glucose metabolism outcomes in the multivariate analysis. However, an overall pattern of decreasing DNRA and NR activities with increasing levels of HOMA-IR was observed under all conditions (Figure 1).
Table 3.
Associations of clinical markers of glucose metabolism with DNRA and nitrate reductase activities in saliva samples.
| Outcome | Exposure | Model | 2nd vs 1st tertile Coefficient (95% CI) |
3rd vs 1st tertile Coefficient (p-value) |
Other Significant Exposures |
|---|---|---|---|---|---|
| HOMA-IR | DNRA_ae | Age, sex | −0.20 (−0.57, 0.18) | -0.50 (−0.87, −0.12)** | |
| Comprehensive | −0.06 (−0.42, 0.31) | -0.48 (−0.81, −0.15)** | Age: 0.03 (0.006, 0.051)*; Smoker-current: −0.45 (−0.76, −0.15)**; BMI: 0.05 (0.03, 0.07)*** | ||
| DNRA_co2 | Age, sex | −0.08 (−0.41, 0.25) | -0.50 (−0.79, −0.21)*** | Age: −0.04 (−0.05, −0.02)***; Sex: -0.30 (-0.57, -0.03)* | |
| Comprehensive | 0.04 (−0.25, 0.33) | -0.42 (−0.68, −0.17)*** | Smoker-current: 0.34 (0.07, 0.61)*; BMI: 0.04 (0.02, 0.06)*** | ||
| NR_ae | Age, sex | 0.14 (−0.23, 0.52) | 0.03 (−0.40, 0.47) | ||
| Comprehensive | 0.09 (−0.30, 0.47) | −0.10 (−0.43, 0.24) | Sex: −0.31 (−0.62, −0.002)*; BMI: 0.06 (0.04, 0.09)*** | ||
| NR_co2 | Age, sex | −0.09 (−0.43, 0.25) | −0.25 (−0.63, 0.14) | Age: −0.03 (−0.05, −0.01)*** | |
| Comprehensive | 0.10 (−0.18, 0.37) | −0.01 (−0.34, 0.33) | BMI: 0.05 (0.03, 0.07)*** | ||
| HbA1c | DNRA_ae | Age, sex | -0.054 (−0.105, −0.003)* | −0.045 (−0.110, 0.021) | |
| Comprehensive | −0.051 (−0.107, 0.004) | −0.051 (−0.117, 0.016) | |||
| DNRA_co2 | Age, sex | −0.022 (−0.065, 0.021) | −0.026 (−0.062, 0.011) | ||
| Comprehensive | −0.023 (−0.067, 0.020) | −0.026 (−0.064, 0.012) | |||
| NR_ae | Age, sex | 0.049 (−0.032, 0.129) | 0.013 (−0.055, 0.081) | ||
| Comprehensive | 0.043 (−0.041, 0.127) | 0.015 (−0.048, 0.077) | |||
| NR_co2 | Age, sex | −0.014 (−0.055, 0.028) | −0.003 (−0.042, 0.036) | ||
| Comprehensive | −0.009 (−0.061, 0.043) | 0.006 (−0.048, 0.059) | |||
| Fasting glucose | DNRA_ae | Age, sex | −0.105 (−0.217, 0.008) | -0.134 (−0.255, −0.013)* | |
| Comprehensive | −0.102 (−0.216, 0.011) | -0.144 (−0.268, −0.019)* | Smoker-current: −0.11 (−0.21, −0.003)*; | ||
| DNRA_co2 | Age, sex | -0.064 (−0.127, −0.0007)* | -0.070 (−0.130, −0.011)* | Sex: −0.051 (−0.099, −0.002)* | |
| Comprehensive | −0.049 (−0.109, 0.010) | −0.054 (−0.116, 0.009) | BMI: 0.006 (0.002, 0.009)** | ||
| NR_ae | Age, sex | 0.09 (−0.07, 0.25) | −0.04 (−0.16, 0.08) | ||
| Comprehensive | 0.08 (−0.08, 0.24) | −0.04 (−0.14, 0.07) | |||
| NR_co2 | Age, sex | −0.05 (−0.11, 0.02) | −0.03 (−0.10, 0.03) | ||
| Comprehensive | −0.03 (−0.10, 0.04) | −0.01 (−0.08, 0.06) | Smoker-former: −0.07 (−0.14, −0.0008)*; BMI: 0.006 (0.002, 0.011)** | ||
| 2-h glucose | DNRA_ae | Age, sex | −0.06 (−0.32, 0.19) | −0.03 (−0.19, 0.13) | |
| Comprehensive | −0.05 (−0.31, 0.20) | −0.02 (−0.18, 0.15) | |||
| DNRA_co2 | Age, sex | −0.09 (−0.27, 0.09) | -0.24 (−0.40, −0.07)** | ||
| Comprehensive | −0.07 (−0.26, 0.12) | -0.21 (−0.37, −0.04)* | |||
| NR_ae | Age, sex | 0.01 (−0.19, 0.21) | −0.1 (−0.27, 0.07) | ||
| Comprehensive | −0.01 (−0.20, 0.19) | −0.1 (−0.27, 0.06) | |||
| NR_co2 | Age, sex | −0.12 (−0.32, 0.07) | −0.11 (−0.30, 0.07) | ||
| Comprehensive | −0.04 (−0.25, 0.16) | −0.002 (−0.20, 0.20) | METs: −0.002 (−0.004, −0.0004)* |
DNRA: nmolesNH4+/min/mg; NR: nmoles NO2−/min/mg; ae: aerobic incubation, co2: incubation in CO2-enriched (reduced oxygen) conditions. Continuous health outcomes were used in Gamma regression models. Comprehensive model has covariates, including age, sex, smoking, BMI, and METs (physical activity). p-values: *<0.05; **<0.01; ***<0.001.
Figure 1.
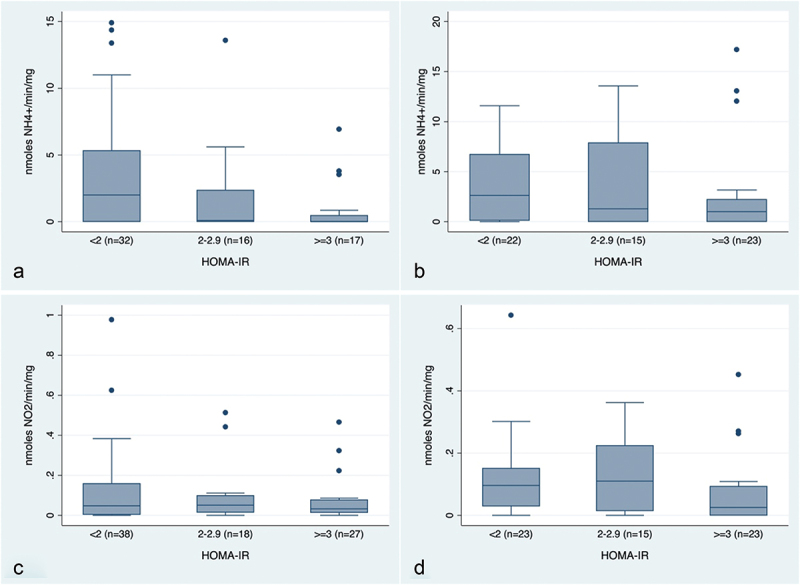
DNRA (nmoles NH4+/min/mg) and nitrate reductase (NO2−/min/mg) activity of saliva samples by HOMA-IR categories under aerobic (a, c) and CO2-enriched (reduced-oxygen) conditions (b, d). HOMA-IR categories have been defined according to proposed thresholds for diabetes (<2: Normal, 2–2.9: Pre-diabetes, ≥3: Diabetes) [18–20].
Discussion
The reduction of nitrate by oral bacteria generates nitrite, ammonium, and NO, and increases the oral pH. For this reason, there has been a growing interest in clinical applications of nitrate and nitrate-reducing bacteria as probiotics/symbiotics to promote cardiometabolic and oral health [21–25]. Previous studies have shown associations between nitrate-reducing oral bacteria and predicted pathways in samples from subgingival dental plaque or from the tongue with cardiometabolic outcomes [4,26,27]. Our study demonstrates directly that the capacity of salivary bacteria to reduce nitrate is inversely associated with insulin resistance, one of the hallmarks in the development of type 2 diabetes and other metabolic disorders [28]. By interpreting the coefficients in Table 3, our data suggest up to 40% lower HOMA-ir levels in the participants with the highest levels of DNRA activity in their saliva (3rd quartile) compared to those in the 1st quartile. Significant inverse associations were also observed between DNRA and other clinical parameters of glucose metabolism, such as blood glucose levels and HbA1c. Furthermore, our findings suggest that lifestyle and host factors, such as oral hygiene practices, smoking, physical activity, BMI and the availability of sugars or nitrates in the mouth, could significantly impact nitrate reduction in oral bacteria and, consequently, cardiometabolic health.
NO metabolism in humans involves an intricate nexus of interconnected human and microbial pathways, which are tightly linked to glucose metabolism (Figure 2). Consequently, type 2 diabetes has been associated with impaired NO metabolism and bioavailability, in part due to suppressed synthesis via both the L-arginine-eNOS pathway and the entero-salivary pathway [3,21]. As shown in Figure 2, insulin signaling activates endothelial nitric oxide synthase (eNOS) by phosphorylation via the PI3K-Akt pathway, thus stimulating endogenous NO production from L-arginine [29]. Increased endothelial NO levels cause vasodilation, which increases the delivery of insulin and glucose to skeletal muscle and fat, facilitating insulin-mediated glucose uptake. Conversely, a deficiency of endothelial NO impairs the vascular distribution of insulin to the tissues. It also affects mitochondrial biogenesis, creating a vicious cycle that may lead to metabolic abnormalities, including insulin resistance [2]. Increased NO synthesis also increases salivary nitrate levels through the entero-salivary pathway [1]. Increased nitrate levels in the saliva induce changes in the oral microbial community, associated with a higher capacity to reduce nitrate and increased NO-bioavailability [23,24]. This model can explain the significant inverse association between the production of ammonium from nitrate in salivary bacteria and insulin resistance observed in our study. Our findings support the hypothesis that measuring the production of ammonium from nitrate by salivary bacteria could be applied to develop novel, non-invasive methods for monitoring NO metabolism and insulin signaling using saliva samples.
Figure 2.
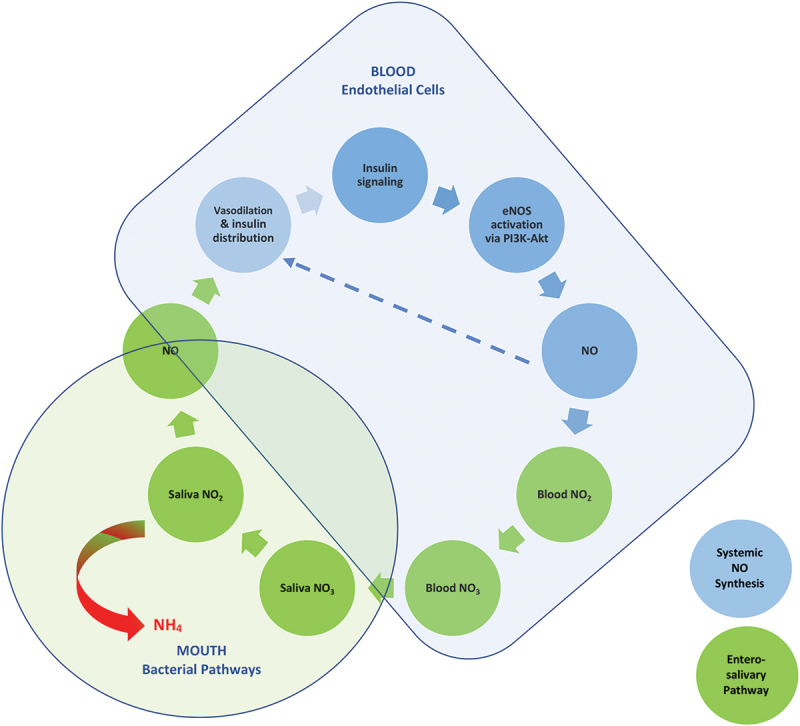
Ammonium production from oral nitrate reduction as a biomarker of the entero-salivary pathway and insulin signaling.
Our study had important strengths, including the availability of detailed, high-quality clinical and lifestyle data and saliva samples from SOALS. Studies on nitrate-reducing oral bacteria typically focus on the tongue microbiome and other anaerobic areas of the mouth (i.e. subgingival dental plaque) as the key oral niches for nitrate reduction; however, a recent meta-analysis using data from the Oral Microbiome Project demonstrated that saliva carries the highest proportions of nitrate-reducing bacteria in the mouth [30]. All samples were collected, processed, and stored under carefully monitored standardized conditions, thus minimizing the risk of bias from using frozen specimens. The measurements of nitrate/nitrite in the saliva samples were within expected ranges based on the literature [31,32].
Nitrite production from nitrate by salivary bacteria was not significantly associated with insulin resistance, as was the production of ammonium, although the patterns were similar. Post hoc power estimates from one-way ANOVA confirmed that the study had sufficient power (0.9992) to detect differences in HOMA-IR for NR under aerobic and CO2-enriched conditions (Cohen’s f of 0.158 and 0.186, respectively). The absence of significant associations with NR could be due, in part, to the fact that nitrite is an intermediate product in bacterial reduction pathways. The amounts of nitrite measured in our assay reflect the relative rate of reactions that produce it (nitrate reduction) versus those that convert it to NO (biochemical reduction in hypoxic/acidic environments, bacterial denitrification) or ammonium (DNRA) under specific conditions. The kinetics of these reactions need to be studied in detail to more accurately assess the association of nitrite with clinical outcomes. These assays require larger sample volumes, which were not available in SOALS, and can be complicated by extreme diversity in the types and numbers of nitrate-reducing bacteria and related pathways in saliva samples [30], as well as the different experimental conditions and host-related factors. The lack of detailed kinetic data presents a limitation of this clinical study, especially in the interpretation of the results related to nitrite. Notwithstanding these limitations, we were able to observe significant associations with ammonium, which is a terminal product that can be more accurately measured under experimental conditions. Using this data we can optimize the methodology that will permit a more precise assessment of these pathways in future clinical studies. Ongoing analysis of the salivary microbiome of the SOALS participants will provide more information regarding the specific bacterial populations and pathways associated with oral nitrate reduction, NO metabolism, and cardiometabolic health.
The regulatory mechanisms of nitrate reduction in oral bacteria, particularly those controlling the conversion of nitrite into NO (denitrification) vs. ammonium (DNRA), are largely unexplored. To begin understanding these mechanisms, our study employed an integrated clinical and ecological approach that considered nitrate reduction within the context of the oral environment by assessing different oral conditions (aerobic vs. CO2-enriched), as well as the impact of oral health-related behaviors that may modulate the oral environment. Within the previously discussed limitations of this approach, our findings are consistent with a vast body of literature regarding the universal controls of nitrate reduction pathways in nature. For example, in most natural ecosystems, these pathways are controlled by the availability of carbon and nitrogen source, as well as nitrate-to-nitrite ratio [9,10]. In our study, the addition of sucrose, a common electron donor for nitrate reduction in oral bacteria, was associated with increased nitrate reduction. Dietary nitrate supplementation is known to promote the growth of denitrifying oral bacteria, such as Neisseria and Haemophilus, while reducing DNRA-expressing species, such as Prevotella and Veillonella [24]. In agreement with these observations, in our study, salivary nitrate levels were positively associated with nitrite production, likely due to increased NR and decreased DNRA activities.
Oral hygiene practices such as tongue cleaning have been shown to impact nitrate metabolism in tongue bacteria by promoting aerobic, denitrifying species such as Neisseria [27]. In our study, brushing frequency was positively associated with ammonium production under CO2-enriched conditions. Smoking differentially impacts aerobic vs. anaerobic nitrate-reducing bacteria: aerobic species such as the denitrifying genera Neisseria and Haemophilus are generally reduced in smokers, whereas more anaerobic genera, such as the DNRA expressing Veillonella and Prevotella, generally increase [33]. In accordance with this, our data showed that ammonium production from nitrate in current smokers was reduced under aerobic conditions but increased under CO2-enriched conditions.
Regular mouthwash use (twice/day or more frequently) was associated with a significantly higher risk of developing diabetes/pre-diabetes in the SOALS cohort, which we hypothesized could be due to an impact on nitrate-reducing oral microbiome [7]; however, mouthwash use did not have a significant association with the production of ammonium or nitrite by salivary bacteria in this study. Previous studies have suggested that OTC mouthwash formulations do not have a significant impact on nitrate-reducing oral bacteria [34]. While our present study evaluated nitrate reduction in saliva, mouthwash may have a more significant impact on other oral microbial niches, such as the tongue. Mouthwash has been suggested to differentially impact oral nitrate reduction in human tongue samples depending on the oral hygiene habits of the participants (i.e. tongue cleaning) and the predominant types of nitrate-reducing species present (denitrifiers vs. ammonium producers) [27]. A larger study with more detailed information on the types of mouthwash used and microbial composition will be required to assess more precisely the impact of mouthwash on nitrate reduction in different oral sites with respect to clinical outcomes.
Our data showed significant associations between the capacity of salivary bacteria to reduce nitrate, BMI and physical activity, which, to our knowledge, have not been previously reported. These observations are not surprising because NO is known to play important roles in various pathophysiological processes involved in metabolism, exercise, and energy expenditure through its involvement in endothelial function, inflammation, and mitochondrial biogenesis [35]. Notably, our population consisted of older adults, who were all overweight or obese, conditions that are associated with compromised endothelial function and NO synthesis, and that may have influenced our findings. Recent studies suggest that periodontal disease, a chronic inflammatory disease that is highly prevalent in older adults, can also impact nitrate reduction in oral bacteria [36]; therefore, the periodontal status of the participants should also be considered in future studies. Environmental exposures and pollutants, such as fine particular matter, have recently been linked to the development of insulin resistance through mechanisms related to NO metabolism, such as oxidative stress, inflammation and inhibition of the PI3K-Akt pathway [37,38]; therefore, the physical environment, food environment and diet, and other social determinants of health and life-style factors which have been linked to the development of diabetes and insulin resistance [39] should be considered in future studies to deeper dissect the role of the oral microbiome in the development of diabetes through NO metabolism.
In summary, this study employed an integrated clinical and ecological approach to assess the role of nitrate reduction by the salivary microbiome in cardiometabolic health while adjusting for important confounders. Our findings describe a symbiotic pathway between the human body and its microbiome, which can lead to novel, non-invasive approaches for monitoring the development of insulin resistance and diabetes using saliva. Despite the limitations and challenges of a clinical study, our data also indicate that lifestyle factors, such as oral hygiene and smoking, could modulate nitrate reduction pathways in oral bacteria, potentially impacting NO bioavailability and cardiometabolic health. These observations highlight the urgent need to better characterize the complex mechanisms that regulate nitrate reduction in oral bacteria and translate these findings into effective clinical interventions for improving oral and systemic health.
Acknowledgments
We would like to thank UPR students Joseluis Torres, Adrian Suarez, Cecille Velez, Camilla Febres, Ana Beconsme, Camilla Espada, and Jorge Bermudez for their help with the assays and/or literature review, and Dr Francisco Bermúdez for technical assistance with the nitrite measurements. Dr. Evangelia Morou-Bermúdez: Contributed to conception and design; funding acquisition, analysis and interpretation of data; and drafted and critically revised the manuscript for important intellectual content. Dr. Kai Guo: Contributed to analysis and interpretation of data, and critically revised the manuscript for important intellectual content. Jairelisse Morales: Contributed to acquisition of data, and critically revised the manuscript for important intellectual content. Karina Ricart: Contributed to acquisition of data, and critically revised the manuscript for important intellectual content. Dr. Rakesh P. Patel: Contributed to analysis and interpretation of data, and critically revised the manuscript for important intellectual content. Dr. José C. Clemente: Contributed to the interpretation of data, and critically revised the manuscript for important intellectual content. Dr. Kaumudi Joshipura: Contributed to conception; funding acquisition and interpretation of data; and critically revised the manuscript for important intellectual content.
Funding Statement
The research reported was supported by the National Institute for Dental and Craniofacial Research under awards number [R01DE028195 and R01DE020111] and by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health under award number [U54GM133807]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure statement
Dr. R. Patel is a coinventor on the use of nitrite salts for the treatment of cardiovascular conditions and chronic ischemia and a co-inventor on a provisional patent for methods to diagnose and predict chronic lung and bowel disease in pre-term infants. Dr. Morou-Bermúdez is inventor on a provisional patent application for the use of ammonium production from nitrate by oral bacteria as a biomarker for monitoring insulin resistance filed by the University of Puerto Rico. Other authors do not have any conflicts of interest to report.
Data and materials availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Kapil V, Khambata RS, Jones DA, et al. The noncanonical pathway for in vivo nitric oxide generation: the nitrate-nitrite-nitric oxide pathway Pharmacol Rev. 2020. doi: 10.1124/pr.120.019240 [DOI] [PubMed] [Google Scholar]
- [2].Ghasemi A, Jeddi S.. Anti-obesity and anti-diabetic effects of nitrate and nitrite. Nitric Oxide. 2017;70:9–11. doi: 10.1016/j.niox.2017.08.003 [DOI] [PubMed] [Google Scholar]
- [3].Lundberg JO, Carlström M, Weitzberg E. Metabolic effects of dietary nitrate in health and disease. Cell Metab. 2018;28(1):9–22. doi: 10.1016/j.cmet.2018.06.007 [DOI] [PubMed] [Google Scholar]
- [4].Goh CE, Trinh P, Colombo PC, et al. Association between Nitrate‐Reducing oral bacteria and cardiometabolic outcomes: results from ORIGINS. J Am Heart Assoc. 2019. [cited 2021 April 25];8(23). doi: 10.1161/JAHA.119.013324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wei YS, Hsiao YC, Su GW, et al. Identification of hyperglycemia-associated microbiota alterations in saliva and gingival sulcus. Arch Biochem Biophys. 2020:682. doi: 10.1016/j.abb.2020.108278 [DOI] [PubMed] [Google Scholar]
- [6].Beals JW, Binns SE, Davis JL, et al. Concurrent beet juice and carbohydrate ingestion: influence on glucose tolerance in obese and nonobese adults. J Nutr Metab. 2017. [cited 2019 May 21]:1–7. doi: 10.1155/2017/6436783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Joshipura KJ, Muñoz-Torres FJ, Morou-Bermudez E, et al. Over-the-counter mouthwash use and risk of pre-diabetes/diabetes. Nitric Oxide. 2017. [cited 2018 January 22];71:14–20. doi: 10.1016/j.niox.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kraft B, Strous M, Tegetmeyer HE. Microbial nitrate respiration–genes, enzymes and environmental distribution. J Biotechnol. 2011. [cited 2019 May 21];155(1):104–117. doi: 10.1016/j.jbiotec.2010.12.025 [DOI] [PubMed] [Google Scholar]
- [9].Kraft B, Tegetmeyer HE, Sharma R, et al. Nitrogen cycling. The environmental controls that govern the end product of bacterial nitrate respiration. Science. 2014. [cited 2019 May 21];345(6197):676–679. doi: 10.1126/science.1254070 [DOI] [PubMed] [Google Scholar]
- [10].Pandey CB, Kumar U, Kaviraj M, et al. DNRA: a short-circuit in biological N-cycling to conserve nitrogen in terrestrial ecosystems. Sci Total Environ. 2020. doi: 10.1016/j.scitotenv.2020.139710 [DOI] [PubMed] [Google Scholar]
- [11].Morou-Bermúdez E, Torres-Colón JE, Bermúdez NS, et al. Pathways linking oral bacteria, nitric oxide metabolism, and health. J Dent Res. 2022. [cited 2023 April 30];101(6):623–631. doi: 10.1177/00220345211064571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Andriankaja OM, Jiménez JJ, Muñoz-Torres FJ, et al. Lipid-lowering agents use and systemic and oral inflammation in overweight or obese adult Puerto Ricans: the san juan overweight adults longitudinal study (SOALS). J Clin Periodontol. 2015;42(12):1090–1096. doi: 10.1111/jcpe.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pérez CM, Muñoz F, Andriankaja OM, et al. Cross-sectional associations of impaired glucose metabolism measures with bleeding on probing and periodontitis. J Clin Periodontol. 2017. [cited 2019 August 1];44(2):142–149. doi: 10.1111/jcpe.12662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Grøn P, Messer AC. An investigation of the state of carbon dioxide in human saliva. Arch Oral Biol. 1965;10(5):757–763. doi: 10.1016/0003-9969(65)90129-9 [DOI] [PubMed] [Google Scholar]
- [15].Pleil JD, Ariel Geer Wallace M, Davis MD, et al. The physics of human breathing: flow, timing, volume, and pressure parameters for normal, on-demand, and ventilator respiration. J Breath Res. 2021;15(4):042002. doi: 10.1088/1752-7163/ac2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Morales Morales J, Febres-Cruz C, Bencosme-Velasquez AK, et al. Identification of organic and inorganic electron donors for nitrate reduction in salivary bacteria. In: Biological and biomedical sciences diversity and inclusion collective (YBDIC) research symposium. New Haven, Connecticut; 2022. [Google Scholar]
- [17].Lang JD, Teng X, Chumley P, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Investigation. 2007;117(9). doi: 10.1172/JCI31892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ghasemi A, Tohidi M, Derakhshan A, et al. Cut-off points of homeostasis model assessment of insulin resistance, beta-cell function, and fasting serum insulin to identify future type 2 diabetes: Tehran lipid and glucose study. Acta Diabetol. 2015. [cited 2023 May 30];52(5):905–915. doi: 10.1007/S00592-015-0730-3 [DOI] [PubMed] [Google Scholar]
- [19].Lee CH, Shih AZL, Woo YC, et al. Optimal cut-offs of homeostasis Model assessment of insulin resistance (HOMA-IR) to identify dysglycemia and type 2 diabetes mellitus: a 15-year prospective study in Chinese. PLOS ONE. 2016. [cited 2023 May 30];11(9):e0163424. Available from: https://pubmed.ncbi.nlm.nih.gov/27658115/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Horáková D, Ladislav Š, Janout V, et al. Optimal homeostasis model assessment of insulin resistance (HOMA-IR) cut-offs: a cross-sectional study in the Czech population. Medicina (B Aires). 2019. [cited 2023 May 30];55(5):158. doi: 10.3390/MEDICINA55050158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bahadoran Z, Mirmiran P, Carlström M, et al. Inorganic nitrate: a potential prebiotic for oral microbiota dysbiosis associated with type 2 diabetes. Nitric Oxide. 2021. [cited 2023 May 1];116:38–46. Available from: https://pubmed.ncbi.nlm.nih.gov/34506950/ [DOI] [PubMed] [Google Scholar]
- [22].Rosier BT, Buetas E, Moya-Gonzalvez EM, et al. Nitrate as a potential prebiotic for the oral microbiome. Sci Rep. 2020. [cited 2020 August 12];10(1). doi: 10.1038/s41598-020-69931-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vanhatalo A, Blackwell JR, Je L, et al. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic Biol Med. 2018. [cited 2019 May 21];124:21–30. doi: 10.1016/j.freeradbiomed.2018.05.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vanhatalo A, Je L, Kelly J, et al. Network analysis of nitrate-sensitive oral microbiome reveals interactions with cognitive function and cardiovascular health across dietary interventions. Redox Biol. 2021;41:101933. doi: 10.1016/j.redox.2021.101933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Velmurugan S, Gan JM, Rathod KS, et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr. 2016. [cited 2019 August 10];103(1):25–38. doi: 10.3945/ajcn.115.116244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Goh CE, Bohn B, Marotz C, et al. Nitrite generating and depleting capacity of the oral microbiome and cardiometabolic risk: results from ORIGINS. J Am Heart Assoc. 2022. [cited 2023 April 30];11(10). doi: 10.1161/JAHA.121.023038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tribble GD, Angelov N, Weltman R, et al. Frequency of tongue cleaning impacts the human tongue microbiome composition and enterosalivary circulation of nitrate. Front Cell Infect Microbiol. 2019;9(MAR). doi: 10.3389/fcimb.2019.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Al-Badrani SM, Al-Sowayan NS. Consequences of insulin resistance long term in the body and its association with the development of chronic diseases. J Biosci Med (Irvine). 2022;10(12):96–109. doi: 10.4236/jbm.2022.1012009 [DOI] [Google Scholar]
- [29].Fisslthaler B, Benzing T, Busse R, et al. Insulin enhances the expression of the endothelial nitric oxide synthase in native endothelial cells: a dual role for akt and AP-1. Nitric Oxide. 2003;8(4):253–261. doi: 10.1016/S1089-8603(03)00042-9 [DOI] [PubMed] [Google Scholar]
- [30].L’heureux Id JE, Van Der Giezen Id M, Winyard Id PG, et al. Localisation of nitrate-reducing and highly abundant microbial communities in the oral cavity. PLOS ONE. 2023;18(12):e0295058. doi: 10.1371/journal.pone.0295058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-X [DOI] [PubMed] [Google Scholar]
- [32].Tenovuo J. The biochemistry of nitrates, nitrites, nitrosamines and other potential carcinogens in human saliva. J Oral Pathol. 1986. [cited 2019 May 21];15(6):303–307. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3093650 [DOI] [PubMed] [Google Scholar]
- [33].Maki KA, Ganesan SM, Meeks B, et al. The role of the oral microbiome in smoking-related cardiovascular risk: a review of the literature exploring mechanisms and pathways. J Transl Med. 2022. [cited 2024 November 20];20(1). doi: 10.1186/s12967-022-03785-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mitsui T, Harasawa R. The effects of essential oil, povidone-iodine, and chlorhexidine mouthwash on salivary nitrate/nitrite and nitrate-reducing bacteria. J Oral Sci. 2017. [cited 2019 August 11];59(4):597–601. doi: 10.2334/josnusd.16-0593 [DOI] [PubMed] [Google Scholar]
- [35].Knott AB, Bossy-Wetzel E. Impact of nitric oxide on metabolism in health and age-related disease. Diabetes Obes Metab. 2010;12(SUPPL. 2):126–133. doi: 10.1111/j.1463-1326.2010.01267.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rosier BT, Johnston W, Carda-Diéguez M, et al. Nitrate reduction capacity of the oral microbiota is impaired in periodontitis: potential implications for systemic nitric oxide availability. Int J Oral Sci. 2024;16(1). doi: 10.1038/s41368-023-00266-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dolcini J, Landi R, Ponzio E, et al. Association between tnf-α, cortisol levels, and exposure to PM10 and PM2.5: a pilot study. Environ Sci Eur. 2024. [cited 2025 March 21];36(1):1–16. doi: 10.1186/s12302-024-00961-2 [DOI] [Google Scholar]
- [38].Lu Y, Qiu W, Liao R, et al. Subacute PM2.5 exposure induces hepatic insulin resistance through inflammation and oxidative stress. Int J Mol Sci. 2025. [cited 2025 March 22];26(2):812. doi: 10.3390/ijms26020812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sung K, Lee S. Social determinants of health and type 2 diabetes in Asia. J Diabetes Investig. 2025. Mar 18 [cited 2025 March 22]. doi: 10.1111/jdi.70024 [DOI] [PMC free article] [PubMed] [Google Scholar]


