Abstract
Lead-free halide double perovskites (HDP) have attracted enormous attention in recent years due to their low toxicity, excellent stability, tunable optical properties, and extensive range of compositional possibilities they present. In the very broad HDP family, Ag-based materials are of particular interest due to their easy synthesis, stability to light and moisture, and interesting optical properties, especially in the form of nanocrystals. Given the very large compositional space, theoretical studies play a crucial role in providing insights into the most promising dopant and possible defect interactions to guide the synthesis and explain the properties. In this review, we discuss recent theoretical works on Ag-based perovskites with an emphasis on density functional theory (DFT) calculations. The computational methods and tools are evaluated, assessing their relative strengths and limitations in their ability to clarify experimental results. We focus specifically on how lattice defects influence the structure and properties of HDP, including lattice and thermodynamic stability, band gap tuning, and photoluminescence.
Keywords: halide double perovskites, Ag-based materials, nanocrystals, stability, computational methods
Introduction
Over the past decade, the field of lead halide perovskites (LHP) has seen significant advancements, particularly in optoelectronic applications such as solar cells, photodetectors, and light-emitting devices.1−3 The general chemical formula for these materials is ABX3, where A represents a monovalent cation, B the divalent cation Pb, and X a halide. This structure has paved the way to the investigation and development of many different Pb-based halides with a diverse range of properties, including high absorption in the visible light spectrum,4,5 tunable band gaps,2,4 and remarkable charge-carrier mobilities.6 However, the use of LHP is limited by the inherent toxicity of Pb7 and their instability under ambient conditions.8 These challenges have driven the exploration of lead-free alternatives.
The initial investigations into the search for lead-free perovskites primarily focused on substituting Pb2+ with isovalent cations such as Sn2+ or Ge2+, but these materials often suffer from rapid oxidation and generally poor stability.9,10 A promising alternative involves replacing Pb2+ with a monovalent cation and a trivalent cation, resulting in the ordered halide double perovskite (HDP) structure known as the elpasolite. HDP have general formula A2B′B″X6, where A is a monovalent cation, B′ and B″ are alternating monovalent and trivalent cations in the B-site, and X represents halide anions such as Cl, Br, or I. The HDP therefore maintains the overall perovskite structure while allowing for the incorporation of other elements less toxic than lead.5,10,11 Still displaying relatively lower power conversion efficiencies (PCEs) compared to Pb-based perovskites, these materials exhibit much higher environmental stability, and a significantly lower toxicity.12−14
Among HDP, those with Ag as a monovalent cation, Cs2AgB″X6, have been the subject of extensive and ongoing research in the recent literature. They have emerged as promising candidates due to their high stability and long carrier lifetimes.10−13,15 These HDP all show a cubic perovskite arrangement, with [AgX6] and [B″X6] octahedra connected at the corners, but the structural and optical properties can be controlled by varying the composition of the trivalent cation and anion sites. An example of such control concerns the direct or indirect character of the transitions, which can be altered by substituting the trivalent cation.14,16 Additionally, Ag-based HDP can achieve excellent photoluminescence properties and improved quantum yields through Na+ alloying and Bi3+ doping.17 These examples show the significant potential of this class of HDP for a range of prospected optoelectronic applications, including solar cells, photodetectors, and light-emitting diodes (LEDs).11−13
Computational methods have played a crucial role in understanding and optimizing the properties of HDP in recent years. These computational methods have been essential for studying the extensive family of HDP, employing tolerance factors and first-principles calculations based on density functional theory (DFT), which have facilitated the systematic evaluation of structural, electronic, and transport properties. Through DFT calculations, valuable information has been collected, and databases have been generated containing data about optical absorption, conversion efficiency, phonon stability, and parameters such as energy above the hull, band gaps, and electron–hole effective masses. These resources facilitate the screening and identification of promising HDP candidates for photovoltaic applications.18 Among the most promising candidates identified through theoretical calculations, those containing Ag have been particularly recognized for their thermodynamic stability, high defect tolerance, and optimal properties as solar absorbers.18−23
The synergy between theoretical and experimental research is essential to address the remaining challenges and unlock the full potential of HDP in any optoelectronic application. Currently, a wide variety of competing DFT softwares are available, each with its own implementations, strengths, and limitations, depending on the system being studied. For HDP systems, the most widely used software packages are the Vienna Ab-initio Simulation Package (VASP),24 ABINIT,25 WIEN2k,26 the Cambridge Serial Total Energy Package (CASTEP),27 and Quantum Espresso (QE).28 Due to such diversity, the prediction of HDP electronic properties may vary between studies, and a proper framing of the different approaches is necessary in order to rationalize their results.
In general, the first difference when the electronic properties of materials with DFT are evaluated arises from the choice of the exchange-correlation (XC) functional. This must be guided by a balance between acceptable computational cost and the required level of accuracy. Less computationally demanding functionals, such as local density approximation (LDA),29 almost always underestimate the band gap, but they also offer a general overview of the behavior of a given structure which can often be acceptable. Similarly, Generalized Gradient Approximation (GGA) functionals30 tend to yield underestimated band gap values, yet they provide a more accurate representation of the band structure. Hybrid functionals31 eventually give band structures that are very close to experimental observations by inserting a fraction of exact exchange from the Hartree–Fock theory, although this approach incurs a significantly higher computational expense and a somewhat arbitrary choice of the exact exchange mixing. Other methods alongside DFT, such as Many-Body Perturbation Theory with the GW approach,32,33 have also been employed for the study of the electronic properties of HDP, providing very accurate results, but their computational cost is significantly higher.
In this review, we summarize recent progress in the modeling and understanding of the chemical and photophysical properties of Ag-based HDP using ab initio techniques. The results discussed here were obtained with several different DFT software packages and XC functionals. Differences, similarities, and their alignment with experimental data are all analyzed to evaluate their accuracy in modeling HDP. We focus on outputs related to composition, doping, and vacancies concerning structural stability and electronic characteristics. Additionally, we highlight the current challenges and future opportunities in the field, aiming to provide a comprehensive understanding of DFT methods in studying systems such as double perovskites for advancing sustainable and efficient optoelectronic technologies.
Crystal Structure and Stability
Bulk Structure
HDP with formula A2B′B″X6 usually feature an alternating arrangement of corner-sharing B″X6 and B′X6 octahedra. These structures adopt a face-centered cubic (fcc) arrangement within space group Fm3̅m (No. 225), whose primitive cell is depicted in Figure 1a. In this structure, the A atoms are placed at Wyckoff position 8c, B′ atoms at 4b, B″ atoms at 4a, and X atoms occupy the 24e position. The alternating arrangement of octahedra in the two different B-sites can be perfectly ordered (Figure 1b) as in the mineral elpasolite, randomly distributed (with a space group Pm3̅m resembling a simple perovskite solid solution, Figure 1c), or layered (Figure 1d), although the latter is rarely observed in HDP. As is discussed in the following sections, the degree of order at the B-site plays a crucial role in determining the optical properties of HDP. For simulations of the elpasolite structure, a small unit cell with 10 atoms can be defined for the elpasolite ordered arrangement (Figure 1a). For disordered or layered structures, the unit cell increases to 40 atoms or more, making the computational study more demanding. This also applies to the study of doping, alloying, or other point defects, as the supercell size increases significantly.
Figure 1.
(a) HDP fcc primitive unit cell. Possible arrangements of monovalent and trivalent cations in the B-site of a HDP: (b) ordered (elpasolite); (c) disordered (one of many different arrangements is shown); (d) layered.
Among all known double perovskites crystallizing in the space group Fm3̅m at room temperature (regardless of thermodynamic stability toward decomposition) we can find combinations of 7 elements as A-site cations, 8 as B′-site cations, 34 as B″ cations, and 5 as X-site anions, leading to approximately 9,520 possible combinations.5 This number is even higher when considering possible modifications due to alloying, doping, or vacancy defects given that each of these often changes the properties of the HDP by a great extent. Thanks to DFT methods and machine learning approaches,34,35 the selection of materials can in principle be narrowed down to those with the best properties. Within the most promising compositions, those based on Ag were identified almost one decade ago. These exhibit a wide range of band gaps, from 1.57 to 3.33 eV,36−39 with values suitable for various optoelectronic applications. Furthermore, Ag-based perovskites demonstrate excellent stability under ambient conditions of heat and moisture, along with good mechanical stability.40 Thanks to their relatively easy synthesis, they can be produced in the form of large single crystals,41 nanocrystals,42,43 and films.44,45 However, significant efforts are still required to address the low photoluminescence reported in these materials, which limits their practical applications.5,11,13
Stability
To evaluate theoretically the stability of HDP materials, both crystallographic and thermodynamic considerations are commonly used. Crystallographic factors are important in the early stages of material discovery, as they enable the rapid identification of materials that, based on their atomic size and radii, are geometrically plausible for forming the elpasolite structure, narrowing the material search space. However, their accuracy is limited as they do not account for any electronic effect beyond simple hard sphere packing of ions. In this regard, thermodynamic considerations provide a more comprehensive understanding of the stability. In the following sections, we describe the crystallographic stability of Ag-HDP materials and further complement these studies with thermodynamic analyses, including formation energies and decomposition enthalpies, to provide a more detailed characterization of HDP stability.
Crystallographic Stability
Crystallographic stability is typically analyzed based on Goldschmidt tolerance factor (t), the octahedral factor (μ), and a recently proposed new factor, τ,40,46 that takes into account the oxidation states of the A cation (nA). These stability factors are defined respectively as
| 1 |
| 2 |
 |
3 |
with rA, r(B′),  , and rX being the ionic radius of the corresponding HPD cations and
anions. Normally, Shannon ionic radii are used in these calculations.
The corresponding limits for stable HDP are reported alongside the
respective eqs 1–3. The tolerance factors described above are an adaptation
of the factors proposed for simple perovskites. By a simple averaging
the ionic radii r(B′) and
, and rX being the ionic radius of the corresponding HPD cations and
anions. Normally, Shannon ionic radii are used in these calculations.
The corresponding limits for stable HDP are reported alongside the
respective eqs 1–3. The tolerance factors described above are an adaptation
of the factors proposed for simple perovskites. By a simple averaging
the ionic radii r(B′) and 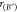 , the corresponding term for the ionic radius rB in the simple perovskite
is replaced. Of course, this can present an inaccurate description
for the case of disordered HDP. Due to a nonhomogeneous distribution
of B′X6–B″X6 octahedra, a simple
average of ionic radii may be insufficient in describing the local
deviations from the average crystal structure. Additionally, these
factors are limited in their application when considering doping or
vacancy defects, whose effect might be either localized or spread
through a larger volume. In the case of alloys, as combinations of
different cations or anions, averages of atomic radii can be considered.
For example, in ref (20), the average ionic radius rX for Cs2AgSb(Br6–xClx) is taken simply as rX = {[(6 – x)rBr + xrCl]/6}, (0
< x < 6). This suggests a linear relationship
between the limits of pure chlorine and pure bromine in terms of the
crystallographic stability factors, assuming the so-called Vegard
law is valid (i.e., the steric effect of alloying is spread throughout
the lattice).
, the corresponding term for the ionic radius rB in the simple perovskite
is replaced. Of course, this can present an inaccurate description
for the case of disordered HDP. Due to a nonhomogeneous distribution
of B′X6–B″X6 octahedra, a simple
average of ionic radii may be insufficient in describing the local
deviations from the average crystal structure. Additionally, these
factors are limited in their application when considering doping or
vacancy defects, whose effect might be either localized or spread
through a larger volume. In the case of alloys, as combinations of
different cations or anions, averages of atomic radii can be considered.
For example, in ref (20), the average ionic radius rX for Cs2AgSb(Br6–xClx) is taken simply as rX = {[(6 – x)rBr + xrCl]/6}, (0
< x < 6). This suggests a linear relationship
between the limits of pure chlorine and pure bromine in terms of the
crystallographic stability factors, assuming the so-called Vegard
law is valid (i.e., the steric effect of alloying is spread throughout
the lattice).
Figure 2 presents a summary of the crystallographic factors reported for Ag-based HDP. All perovskites fall within the stability limits defined by the t-factor. As the ionic radius rX increases, the t-factor deviates further from the ideal value of t = 1 (ideal cubic structure). This is evident when comparing the perovskites to Cl, Br, and I in Figure 2a. As the ionic size increases down the halide group, the t-factor is higher for Cl compounds and lower for I compounds. The values of the octahedral factor (μ) are shown in Figure 2b. All of the HDP fall within the stability limits except for Cs2AgCrI6, suggesting its thermodynamic instability and the difficulty of its possible synthesis. Several Ag-HDP predicted stable by both the t and octahedral factors have, however, been found to be unstable, presenting significant experimental challenges, as is generally the case of iodide HDP.54,55 The main challenge associated with iodide is its large atomic radius (2.2 Å), which requires selecting a suitable trivalent cation to fulfill the criteria of the tolerance factor (t) and (μ) in order to form a stable HDP. With an appropriate combination of the tolerance factor and the radius ratio of trivalent cations, some iodine-based double perovskites have been theoretically proposed to be stable.54 Several of these materials, including Cs2Na(Ce, Nd, Gd, Tb, Dy)I656 and Cs2Ti(Br,I)6,57 have recently been synthesized experimentally, thereby expanding the family of HDP and opening new directions for scientific investigation.
Figure 2.

Reported stability from (a) t-factor, (b) octahedral μ-factor, and (c) τ-factor for Ag-based HDP. The gray areas fall outside the stability limits. Taken from refs (40) (blue), (47) (white), (48) (red), (39) (green), (49) (black), (50) (dark blue), (51) (brown) and (52), and (53) (orange). Some missing values were not found in the literature.
Factors such as τ are, therefore, being explored to improve stability predictions. Indeed, the τ factor predicts the instability of compounds with I, as shown in Figure 2c. Overall, the geometrical stability factors t, μ, and τ may provide a first general assessment of the stability of HDP compositions in terms of their proximity to the perovskite aristotype. These crystallographic factors provide a useful first-pass material screening, after which it is necessary to analyze the electronic interactions through thermodynamic factors to more accurately predict the stability of HDP.
Thermodynamic Stability
The thermodynamic stability of HDP can be evaluated with DFT through total energy calculations in terms of formation energy and decomposition enthalpy. The formation energy measures the energy released or absorbed when the compound is formed from its reactants; the more negative its value, the more exothermic the formation and the greater the HDP stability (the opposite is true for positive energies). In the case of pristine HDP, formation energy is generally calculated with respect to the atoms in a pure solid substance as
| 4 |
where 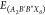 is the total energy of the HDP and EA, EB′, EB″, EX are the corresponding energies of isolated atoms maintaining
the stoichiometry.20,52,55,58−60 The formation energy,
depending on the synthetic pathway, can also be calculated in terms
of precursor compounds and defined as the energy difference between
the HDP and its halide precursors.
is the total energy of the HDP and EA, EB′, EB″, EX are the corresponding energies of isolated atoms maintaining
the stoichiometry.20,52,55,58−60 The formation energy,
depending on the synthetic pathway, can also be calculated in terms
of precursor compounds and defined as the energy difference between
the HDP and its halide precursors.
To assess the relative ease of formation of points defects and alloying in a charge state q, the formation energy is also used, defined as
| 5 |
where EDop and EPrist are the total energies of the defective and pristine supercells, ni and nj are the number of atoms removed and added respectively, μi and μj are the corresponding chemical potentials, and EF is the Fermi energy.61−63
In the literature, the projector augmented wave (PAW) method64 with the GGA65 functionals PBE and PBEsol30 are commonly used for thermodynamic stability calculations. These functionals have been shown to be efficient and provide results in agreement, in terms of formation energy and decomposition enthalpy, with more computationally demanding functionals such as hybrid functionals.21,48,66Table 1 presents the formation energies for various Ag-based HDP, calculated using different DFT packages, i.e., QE, VASP, and Wien2k. Whenever different packages were used, only very small discrepancies were found, up to about 0.02 eV/atom. These small discrepancies are present even when using the same software and functional. They can be attributed to the initial arrangement of atomic positions for subsequent atomic relaxation, force and energy convergence criteria, the number of k-points evaluated, and cutoff energies and densities for the plane waves. All these parameters influence the determination of total energies in systems through DFT, making the evaluation of their convergence essential for obtaining accurate results.
Table 1. Formation Energy of Ag-Based HDP with Respect to Pure Elements.
| HDP | Formation Energy (eV/atom) | Functional | Code | Ref. |
|---|---|---|---|---|
| Cs2AgInBr6 | –1.110 | PBE | VASP | (52) |
| Cs2AgSbBr6 | –1.080 | PBE | VASP | (52) |
| –1.095 | PBE | QE | (58) | |
| –1.107 | PBE | VASP | (20) | |
| Cs2AgBiBr6 | –1.13 | PBE | VASP | (52) |
| –1.136 | PBE | VASP | (55) | |
| –1.156 | PBE | QE | (58) | |
| Cs2AgBiCl6 | –1.370 | PBE | QE | (58) |
| Cs2AgSbCl6 | –1.308 | PBE | QE | (58) |
| –1.304 | PBE | VASP | (20) | |
| Cs2AgBiI6 | –0.885 | PBE | QE | (58) |
| Cs2AgSbI6 | –0.827 | PBE | QE | (58) |
| Cs2AgCrCl6 | –1.000 | PBEsol | Wien2K | (59) |
| Cs2AgCrBr6 | –1.410 | PBEsol | Wien2K | (59) |
| Cs2AgCrI6 | –1.410 | PBEsol | Wien2K | (59) |
| Cs2AgScCl6 | –1.805 | PBE | VASP | (51) |
| Cs2AgScBr6 | –1.539 | PBE | VASP | (51) |
| Cs2AgScI6 | –1.191 | PBE | VASP | (51) |
| Cs2AgFeCl6 | –1.205 | PBE | VASP | (51) |
| Cs2AgFeBr6 | –0.983 | PBE | VASP | (51) |
| Cs2AgFeI6 | –0.690 | PBE | VASP | (51) |
| Cs2AgGaBr6 | –1.080 | PBE | VASP | (52) |
According to Table 1, all HDP have negative formation energies, indicating their thermodynamic stability with respect to the elements. However, some iodine HDP have higher energies, which suggests lower stability compared with the other halides. This is consistent with the tolerance factor, τ, which deviates more from unity in iodides. It is worth noting that the perovskites with the greatest stability – i.e. lowest formation energy, are those containing CrBr and CrI. Nonetheless, these types of perovskites have only been proposed theoretically, and their synthesis has yet to be reported. This suggests the need for a more comprehensive analysis, not only in terms of formation energy but also considering other properties such as decomposition enthalpies and electronic stability. HPD such as Cs2AgBiBr6,67−69 alloys of Cs2AgInBr6, Cs2AgSbBr6,70 Cs2AgBiCl6,71 Cs2AgSbCl6,15,38,72,73 Cs2AgFe(Cl,Br)6,74,75 and rare earth materials as Cs2AgScCl676 have been reported experimentally confirming the predictions made by the formation energy calculations. It is worth also mentioning that Cs2AgInCl611 was successfully synthesized in several studies, but its formation energy is not reported here.
Another measure of the stability of HDP is the decomposition enthalpy ΔH, defined as the difference between the total energy of the decomposed products [E(product)] and the total energy of the HDP:21,40,48,55,58,62
| 6 |
Since HDP contains multiple cations, it exhibits a wide range of decomposition pathways into binary and ternary compounds. In eq 6, parameters a–e and i–n depend on the specific stoichiometry of the ternary compounds. The enthalpy of decomposition can be calculated either for binary compounds alone (with d = e = 0) or for both binary and ternary compounds. The more positive the decomposition enthalpy, the lower the probability of decomposition; the opposite occurs for negative values. According to the literature, the most probable decomposition pathways of Ag-based HDP are
| 7 |
| 8 |
| 9 |
| 10 |
Pathway PA is the complete decomposition to the simplest binary compounds only, while PB also involves the formation to the most stable ternary compound. Since Cs–Ag-X compounds also exist, their formation is accounted for in pathways PC and PD. In Tables 2–4, we present the decomposition enthalpies, the corresponding decomposition pathways, and the code used.
Table 2. Decomposition Enthalpy of AgBr-Based HDPa.
| HDP | Pathway | ΔH (meV/atom) | Code | Ref. |
|---|---|---|---|---|
| Cs2AgBiBr6 | PA | 64, 42, 66.5 | VASP | (55, 52, 21) |
| 41.9 | QE | (58) | ||
| PB | 24, 30, 14.8 | VASP | (55, 52, 21) | |
| 3.9 | QE | (58) | ||
| PC | 16, 5.0 | VASP | (55, 21) | |
| 0.1 | QE | (58) | ||
| PD | 11, 3.9 | VASP | (55, 21) | |
| 1.1 | QE | (58) | ||
| Cs2AgSbBr6 | PA | 62 | VASP | (52) |
| 54.7 | QE | (58) | ||
| PB | –30 | VASP | (52) | |
| –3.9 | QE | (58) | ||
| PC | –7.7 | QE | (58) | |
| PD | –6.7 | QE | (58) | |
| Cs2AgInBr6 | PA | 30, 55.8 | VASP | (52, 21) |
| PB | 22, 0.3 | VASP | (52, 21) | |
| PC | –9.5 | VASP | (21) | |
| PD | –10.5 | VASP | (21) | |
| Cs2AgGaBr6 | PA | –18 | VASP | (52) |
| PB | 9 | VASP | (52) |
References are listed in the same order as the respective decomposition enthalpy values. All decomposition enthalpies were calculated with the PBE functional.
Table 4. Decomposition Enthalpy of AgI-Based HDPa.
| HDP | Pathway | ΔH (meV/atom) | Code | Ref. |
|---|---|---|---|---|
| Cs2AgBiI6 | PA | 1.2 | QE | (58) |
| 9.5 | VASP | (21) | ||
| PB | –22 | QE | (58) | |
| –22.8 | VASP | (21) | ||
| PC | –23.9 | QE | (58) | |
| –28.1 | VASP | (21) | ||
| PD | –19.1 | QE | (58) | |
| Cs2AgSbI6 | PA | 15.7 | VASP | (21) |
| PB | –22.5 | VASP | (21) | |
| PC | –27.8 | VASP | (21) | |
| Cs2AgInI6 | PA | 2.6 | VASP | (21) |
| Cs2AgI3 + InI3 | –18.6 | VASP | (21) | |
| CsI + AgI + CsInI4 | –43.1 | VASP | (21) |
The different pathways for Cs2AgInI6 presented were shown to be the most probable decomposition pathways for this HDP. All decomposition enthalpies were calculated with the PBE functional.
Inspecting the decomposition enthalpy to halides in Tables 2–4, it can be seen that for most Ag-based HDP, the decomposition enthalpy toward the binary compounds alone is higher compared to the other decomposition pathways. Except for the iodide and Ga perovskites, all values for the binary decomposition enthalpy are on the order of tens of meV/atom, sometimes reaching hundreds (Tables 2–3), indicating that it is unlikely for the HDP to decompose into binary compounds. In the case of perovskites with iodine (Table 4), although they have positive decomposition enthalpies for binary decomposition, their values are very small. In fact, they exhibit negative decomposition enthalpies for all other decomposition pathways, showing lower stability compared with other Ag-based perovskites. This confirms the results obtained from the formation energy (see Table 1), τ factor, and the difficulties in achieving their synthesis.
Table 3. Decomposition Enthalpy of AgCl-Based HDPa.
| HDP | Pathway | ΔH(meV/atom) | Code | Ref. |
|---|---|---|---|---|
| Cs2AgBiCl6 | PA | 100 | QE | (58) |
| 121.3 | VASP | (21) | ||
| PB | 21.7 | QE | (58) | |
| 26.7 | VASP | (21) | ||
| PC | 16 | QE | (58) | |
| 16.1 | VASP | (21) | ||
| PD | 14.7 | QE | (58) | |
| 14.6 | VASP | (21) | ||
| Cs2AgSbCl6 | PA | 75.9 | QE | (58) |
| 94.3 | VASP | (21) | ||
| PB | 11.6 | QE | (58) | |
| 15.9 | VASP | (21) | ||
| PC | 5.9 | QE | (58) | |
| 5.3 | VASP | (21) | ||
| PD | 4.5 | QE | (58) | |
| 3.8 | VASP | (21) | ||
| Cs2AgInCl6 | PA | 88.8 | VASP | (21) |
| PB | 18.6 | VASP | (21) | |
| PC | 8.1 | VASP | (21) | |
| PD | 6.6 | VASP | (21) |
All decomposition enthalpies were calculated with the PBE functional.
When considering the decomposition energy into ternary compounds (pathways PB,C,D), the decomposition enthalpy generally decreases for all Ag-based HDP, with some even reaching negative values. This suggests that the perovskite’s stability is compromised, leading to a tendency for decomposition into ternary compounds. Therefore, theoretical works must account for all possible decomposition pathways into binary and ternary compounds to provide an accurate estimation of HDP stability. The perovskites that exhibit the greatest theoretical stability are Cs2AgBiBr6 (Table 2) and all those with Cl (Table 3). Although their decomposition enthalpy is low for some pathways toward ternary compounds, all the values are positive. This fact demonstrates the agreement between theory and experiment, as these perovskites have been synthesized and continue to be extensively studied.
Tables 2–4 show some discrepancies in the decomposition enthalpy values obtained depending on whether the same or different software was used. As mentioned earlier, this can be attributed to slight changes in the input parameters during the execution of the DFT calculations. Despite the discrepancies reaching up to 26 meV/atom in some cases, the positive/negative decomposition enthalpy values remain consistent, which provides insight into whether the material is likely to decompose or remain stable. Clearly, larger absolute values indicate a higher probability of decomposition or stability depending on the sign of the enthalpy. Additionally, the enthalpy values show similar trends when changing the decomposition pathway, when comparing the software packages. Although the results presented are reported by only a few software packages, the trends shown in both the formation energy and decomposition enthalpy exhibit a considerable degree of agreement. It should also be noted that these theoretical results align with experimental findings regarding stability. This validates and motivates the use of DFT methods for predicting the potentially most stable perovskites that can be synthesized.
We must emphasize that to fully assess the HDP thermodynamic stability, it is essential to include, alongside formation energy and decomposition enthalpy, the energy above the convex hull (Ehull). Ehull is an accurate indicator of a compound’s stability, as it is determined by evaluating all possible phase decomposition reactions of the compound.77,78 While Ehull can be calculated using DFT methods, the extensive number of phase decomposition reactions among competing compounds makes its computation highly demanding. However, with recent technological advancements, methodologies such as machine learning (ML) are now being employed to study this type of thermodynamic property in materials. ML algorithms trained with outputs from DFT calculations have shown agreement with both the Ehull values and the experimentally observed stabilities in HDP, and consequently, in Ag-based HDP.77,78 Moreover, ML algorithms have been used to propose new materials as potential stable candidates for synthesis and subsequent optoelectronic applications. ML methods, combined with DFT training sets, have made significant progress in predicting electronic and thermodynamic properties of perovskites and are emerging as valuable auxiliary tools to advance computational and experimental materials science together.34,79
Doping, Alloying and Point Defects
To enhance the stability of perovskites or to control their optical properties, theoretical and experimental methods were employed to explore chemical mixing at the cation and/or anion sites in HDP. Given the feasibility of studying perovskite stability from a theoretical perspective, several studies have proposed that specific configurations with alloying, atomic site changes, and doping result in enhanced stability and optical properties. In ref (58), the decomposition energies for Cs2BiAg1–xCuxCl6, Cs2SbAg1–xCuxCl6, Cs2BiAg(Br1–xIx)6, and Cs2SbAg(Cl1–xBrx)6 (0 < x < 1) were studied, as shown in Figure 3a. Thin lines represent the decomposition energies for various reaction pathways (including binary and ternary compounds), using both LDA29 (blue lines) and PBE functionals (red lines). The thick lines indicate the lowest decomposition energy pathway at each concentration “x”. The results obtained through the PBE show a decreasing trend with a constant rate of change as the concentration “x” increases. The LDA exhibits some variations in the rate of change, and in certain cases, a majority of the values show positive enthalpy. However, it preserves the trend of decreasing stability as the concentration x increases, consistently with PBE. Having mentioned that PBE shows good agreement with experimental results, it may also be added that to some extent the LDA functional reproduces correct trends for the stability of HDP, while giving less reliable total energies. In Figure 3a, the increase in the Cu concentration causes a decrease in the stability of the perovskites. This may be a consequence of Cu preference for 4-fold coordination over 6-fold coordination in the perovskite octahedra, thereby allowing decomposition into ternary compounds.21 As seen in Tables 2 and 4, perovskites with Br exhibit superior stability compared to those with I. Therefore, when Br/I is mixed, a decrease in stability is observed as the concentration of I increases. In the case of Cl/Br mixing, lower concentrations of Br maintain positive decomposition enthalpy values, but as the Br percentage increases, system stability decreases. All of these observations align with the decomposition enthalpy values in Tables 2–4, where Cl-based perovskites generally exhibit greater stability compared to those with Br or I, with I-based perovskites being the least stable.
Figure 3.
(a) Decomposition energy as a function of doping concentration “x” for Cs2BiAg1–xCuxCl6, Cs2SbAg1–xCuxCl6, Cs2BiAg(Br1–xIx)6, and Cs2SbAg(Cl1–xBrx)6. (b) Formation energy of points defects in Cs2AgBiBr6 as a function of the Fermi energy (Ef) under Br-poor (left) and Br-rich (right) conditions. (c) Decomposition enthalpy of doped Cs2AgBiBr6 and Cs2AgBiCl6 and (d) defect formation energy. (e) Relation between stability and dopant formation energy in divalent-doped Cs2AgSbCl6 perovskite. Filled symbols and hollow symbols represent the decomposition toward binary compounds and binary + ternary compounds, respectively. (a) Reprinted with permission from ref (58), Copyright 2018, American Chemical Society. (b) Adapted with permission from ref (55), Copyright 2016 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (c and d) Adapted with permission from ref (80), Copyright 2021, American Chemical Society. (e) Adapted from ref (62) with permission from the Royal Society of Chemistry, Copyright 2022
Xiao et al., in ref (55), conducted a study on 20 point defects in Cs2AgBiBr6 under Br-rich and Br-poor conditions. They investigated four vacancies (VCs, VAg, VBi, and VBr), four interstitials (Csi, Agi, Bii, and Bri), six cation-on-cation antisites (CsAg, CsBi, AgCs, AgBi, BiCs, and BiAg), three cation-on-anion antisites (CsBr, AgBr, and BiBr), and three anion-on-cation antisites (BrCs, BrAg, and BrBi) as a function of the Fermi level (see Figure 3b). Ag vacancies were found to be particularly prone to be generated due to their low formation energy under both Br-rich and Br-poor conditions. Compared to the high defect tolerance of lead-based perovskites, which contain only a divalent cation, and where defects typically manifest as shallow states, located near the valence band maximum (VBM) or conduction band minimum (CBM) with negligible impact on optical properties,13 HDP exhibit substantial differences due to the presence of two distinct B-site cations, leading to the formation of additional antisite defects.81 DFT calculations of thermodynamic transition energy levels were used to understand the role of defects in Ag-HDP. The most easily formed defects are Ag vacancies, which act as shallow acceptors, while Ag interstitials behave as shallow donors. In contrast, B‴ vacancies and Ag/B‴ antisite defects are deep acceptors and become dominant under Br- or Cl-rich conditions. Although HDP exhibit lower defect tolerance compared to lead-based perovskites, synthesis techniques with controlled growth conditions can suppress deep defects under halogen- and B‴-poor or -rich environments, enabling optoelectronic applicability.55,82
In ref (80), the decomposition and defect formation energies of Cs2AgBiCl6 and Cs2AgBiBr6 perovskites doped with M2+ ions from group IV (Sn2+ and Ge2+) and L2– ions from group VI (S2–, Se2–, and Te2–), at a 25% concentration were studied. To calculate stability in terms of decomposition enthalpy toward binary compounds, two doping strategies were considered. First, two M2+ ions replace a monovalent cation Ag+ and a trivalent cation Bi3+ (M2+–M2+ doping), and the second strategy involves an M2+-L2– pair replacing an Ag–(Cl–Br) pair, maintaining charge balance (Figure 3c). To calculate the defect formation energy, the replacement of a Ag site and a Bi site was studied (Figure 3d). One M2+–L2– pair then replaces either a Ag–Cl,Br pair (labeled Ag-replaced) or a Bi–Cl,Br pair (labeled Bi-replaced). Figure 3c shows that the decomposition energies into binary compounds are positive and typically exceed 20 meV/atom, suggesting the potential stability of the doped systems. Nevertheless, further studies on decomposition toward ternary compounds are needed to establish stability under this type of doping. Figure 3d shows positive formation energies, indicating the instability introduced by doping. However, the formation energies are lower when Ag is replaced, implying that potential dopants might be more likely to substitute at the Ag site rather than the Bi site.
The doping with divalent cations in Cs2AgSbCl6 perovskite has also been studied in ref (62). Following the same strategy as before and to maintain charge balance, two divalent cations replace a monovalent cation and a trivalent cation, with doping percentages of 25%. Furthermore, charge compensation with Ag vacancies was also considered. Figure 3e shows the formation energy and decomposition energy toward binary compounds and Cs2Sb3Cl9 plus binary compounds, represented by filled and hollow symbols, respectively. Among the divalent dopant cations, Cd2+ and Zn2+ exhibit low formation energy and positive decomposition energy, indicating the possibility of incorporating this type of doping into the pristine double perovskite.
All in all, we have seen how various DFT methods provide insights into how doping, defects, and alloying affect the stability of double perovskites. Some of them explain why synthesis difficulties arise in certain materials, while others propose new doping strategies that could potentially be applied to future syntheses. Continued refinement of computational models, including the improvement of exchange-correlation functionals and the integration of machine learning techniques, along with the optimization of computational resources through GPU parallelization, is necessary for exploring various compositions to optimize these materials for practical applications. It is important to mention that for a more comprehensive understanding of the stability and synthesis challenges of HDP, an analysis of dynamic stability with respect to temperature, pressure, and deformation should be conducted. Although this particular aspect falls outside the scope of the review, we highlight that dynamical stability has been extensively studied for simple perovskites, but it remains largely unexplored for HDP.
Electronic Structure and Optical Properties
Contrary to expectations, the electronic properties of HDP differ significantly from those of the simple perovskites ABX3. While simple perovskites typically exhibit relatively small direct band gaps, double perovskites often have large indirect band gaps or direct parity-forbidden band gaps with low oscillator strength. Currently, these challenges are being addressed by exploring changes in composition, doping, or alloying. In the following sections, we present the most relevant theoretical results regarding the electronic properties of HDP, and the proposed methods to improve their characteristics as a guide for the design of high-performance HDP.
Band Structure: General Features
Ag-based HDP can be classified into two categories based on their optical properties depending on lone-pair states (s0).16 Type I perovskites have a direct bandgap at the Γ point of the Brillouin zone (see Figure 4a, 4d). For example, in Cs2AgInX6, the VBM is formed by cationic Ag-4d states and anion X-p states, while the CBM is due to antibonding states formed by 5s orbitals of In and Ag, and X-p orbitals. The CBM, which is primarily composed of delocalized In-5s states, along with symmetry inversion, leads to the VBM and CBM having the same even parity, which eventually results in a parity-forbidden direct transition at the Γ point,83 as shown in Figure 4c.
Figure 4.

Electronic bands and states of (a) Type I and (b) Type II HDP. (c) Transition probability between the VBM and CBM for Cs2AgInCl6, with zero probability at the Γ point. (d) First Brillouin zone of the fcc lattice with high symmetry points and paths. (a and c) Reprinted with permission from ref (83), Copyright 2017, American Chemical Society. (b) Adapted with permission from ref (16), Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Type II perovskites, with lone-pair s electrons (s2) on the B″ cation, exhibit indirect band gap with the VBM located at the X point and the CBM at the L point (see Figure 4b). The VBM is primarily composed of Ag-4d, B″-s, and X-p states, while the CBM is mainly formed by B″-p and X-p states.16,83,84 The electronic mismatch in the angular momentum of the atomic orbitals between Ag and B″ in the conduction and valence bands is responsible for the indirect nature of these type of perovskites.5,85 Despite their indirect nature, these perovskites exhibit a relatively smaller band gap between 2.0 and 3.0 eV (which can be tuned through doping or alloying), good stability against heat and moisture, and facile synthesis of crystals, nanocrystals, and films.42,43 All of these features have driven interest in optimizing their optical properties.
Theoretical studies indicate that all Ag-based perovskites can be classified into the two types previously described based on their electronic structure. The shapes of the calculated VBM and CBM for different Ag perovskites remain consistent, with only slight modifications. Among the Ag perovskites, main differences are found in the position of the electronic bands, resulting in different band gap values, as summarized in Table 5. Table 5 provides a comparison of the calculated and experimental band gaps for Ag-based HDP using different exchange-correlation functionals, including the pure GGA PBE and PBEsol functionals, the hybrid HSE,103 the meta-GGA modified Becke–Johnson (mBJ),104 and also the GW method,33 as implemented in VASP, WIEN2k, Quantum Espresso, Yambo,105 and CASTEP.
Table 5. Ag-Based Bandgapsa.
| HDP | Method | Band Gap (eV) | Ref. | Exp. (eV) |
|---|---|---|---|---|
| Cs2AgBiBr6 (Indirect) | HSE | 1.93*V, 2.06 *V, 1.89*V, 1.84*V | (19, 37, 86, 87) | 2.1937 |
| 1.77*V | (88) | 1.989 | ||
| mBJ | 1.94*W, 2.07W | (48) [•] | 2.1890 | |
| PBE | 1.10*W, 1.32W, 1.18*Q, 1.42Q | (48) [•], (91) [•] | ||
| 1.09*V | (86) | |||
| PBEsol | 1.02*W, 1.25W, 1.33Q, 1.10Q | (48) [•], (91), (92) | ||
| G0W0 | 1.8*Y, 2.1*Y | (89), (93) | ||
| Cs2AgBiCl6 (Indirect) | HSE | 2.62*V | (19) | 2.7737 |
| mBJ | 2.35*W, 2.79W | (48) [•] | 2.494 | |
| PBE | 1.49*W, 1.82W, 1.57*Q, 1.91Q | (48) [•],91 [•] | 2.289 | |
| PBEsol | 1.40*W, 1.71W, 1.81Q, 1.71Q | (48) [•], (91, 92) | ||
| G0W0 | 2.40*Y | (89) | ||
| Cs2AgBiI6 (Indirect) | HSE | 1.75*W, 1.33W | (48) [•] | 1.7595 |
| mBJ | 1.30*W, 1.33W, 1.46W | (48) [•], (96) | ||
| PBE | 0.67*Q, 0.89Q, 0.62*W, 0.83W | (91) [•], (48) [•] | ||
| PBEsol | 0.83Q, 0.63*,W, 0.78W | (91), (48) [•] | ||
| Cs2AgInBr6 (Direct) | HSE | 1.47V, 1.50V, 1.64V | (52), (97, 98) | 1.5736 |
| PBE | 0.21V | (52) | ||
| Cs2AgInCl6 (Direct) | HSE | 2.38V, 2.32V, 2.38V, 3.0V | (83), (21), (99), (98) | 3.3339 |
| PBE | 1.03V, 1.00V, 1.03V | (83), (21), (99) | 3.23100 | |
| G0W0 | 3.27V | (17) | 3.2101 | |
| Cs2AgSbBr6 (Indirect) | HSE | 0.88C, 1.67*V, 1.46*V, 1.37*V | (102), (97), (87), (88) | 1.6487 |
| mBJ | 1.5*W, 1.54 W | (48) [•] | ||
| PBE | 0.9C, 0.82*W, 0.85W | (102), (48) [•] | ||
| PBEsol | 0.75*W, 0.78W | (48) [•] | ||
| Cs2AgSbCl6 (Indirect) | HSE | 2.22*V, 1.49C, 2.40*,V | (61, 102, 97) | 2.2894 |
| mBJ | 2.23*W, 2.32W | (48) [•] | 2.7038 | |
| PBE | 1.55C, 1.33*W, 1.40W | (102), (48) [•] | 2.5715 | |
| PBEsol | 1.24*W, 1.30W | (48) [•] | ||
| Cs2AgSbI6 (Indirect) | HSE | 0.41C, 0.95*V | (102, 97) | |
| mBJ | 0.78*W, 0.84W | (48) [•] | ||
| PBE | 0.44C, 0.32*W, 0.39W | (102), (48) [•] | ||
| PBEsol | 0.28*W, 0.35W | (48) [•] | ||
| Cs2AgFeCl6 (Direct) | PBE | 1.17*V, 0.95*V | (102, 97) | |
| mBJ | 0.78*W, 0.84W | (48) [•] | ||
| PBE | 0.44C, 0.32*W, 0.39W | (102), (48) [•] | ||
| PBEsol | 0.28*W, 0.35W | (48) [•] |
V, W, Q, Y, and C stand for VASP, WIEN2k, Quantum Espresso, Yambo, and CASTEP, respectively. (*) means inclusion of SOC effects. References are listed in the same order as for the respective band gap values. The symbol [•] indicates the same reference as the previous one. Exp. stands for the reported experimental values.
The overall electronic structure is consistent across different levels of theory, but differences in the band gap values are significant. For instance, it is well-known that the PBE and PBEsol functionals tend to underestimate band gap values, which can be corrected by using hybrid functionals or through GW methods. However, PBE and PBEsol are reliable for describing electronic states and band morphology while being computationally much less demanding compared to hybrid functionals. The inclusion of spin–orbit coupling (SOC) in HDP generally causes little changes in optical properties.39,83,99,106 The most significant effect of including SOC is reflected in the position of the electron bands, generally resulting in a small decrease in the band gap value. Due to the high computational demand, many theoretical studies on HDP only use SOC to test selected results or ignore it altogether.
Differences in the reported band gap values using the same type of functional appear to be unrelated to the software package employed and can primarily be attributed to calculation parameters such as the size of the k-grid, cutoff energies, and convergence criteria for force, pressure, and energy. Additionally, hybrid functionals are also sensitive to the value of the Hartree–Fock fraction. Using the experimental values in Table 5, hybrid functionals improve the accuracy of bandgaps predicted compared to PBE, but are computationally expensive. mBJ functionals, which use empirical potentials, do not incorporate exact exchange like hybrid functionals, and while they are computationally more efficient, they may also be less accurate. The GW method, grounded in many-body theory, is considered the most accurate for predicting bandgaps but is the most computationally demanding. In summary, mBJ, HSE functionals, and the GW method provide improved bandgap values compared to PBE-type functionals, with increased computational demands proportional to their increased accuracy.
Alloying Effects
Due to the large indirect or direct forbidden bandgap transition in HDP, strategies involving doping, alloying, or introducing disorder are pursued to convert the indirect bandgap into a direct one or to modify the band parity, enhancing their potential for optoelectronic applications.
Du et al. demonstrated bandgap engineering in Cs2AgBiBr6 through In/Sb alloying on Bi site, experimentally confirming an increase in the band gap as the percentage of In increased up to 75% (Cs2AgBi0.25In0.75Br6), and a decrease in the band gap with the inclusion of Sb up to 37.5% (Cs2AgBi0.625Sb0.375Br6), with the smallest band gap value being 1.86 eV. This allowed to lower the band gap of Cs2AgBiBr6 by 0.41 eV. These trends were then studied with HSE+SOC functionals. DFT calculations show that the substitution for Sb introduces 5s states into the VBM with higher energies and 5p states into the CBM (Figure 5b) with energies similar to those in pristine Cs2AgBiBr6 (Figure 5a). These changes results in a band gap decrease, consistent with experimental results.70 Analogous trends with the incorporation of Sb were obtained theoretically through PBE and HSE+SOC.107
Figure 5.
Electronic band structure of (a) Cs2AgBiBr6, (b) Cs2AgBi0.75Sb0.25Br6, (e) Cs2AgBi0.75In0.25Br6, and (f) Cs2AgBi0.25In0.75Br6. (c) Band alignment and bandgap of Cs2AgBiBr6 as a function of disorder degree: fully ordered, partially disordered, and fully disordered. (g) DOS of Cs2AgBiBr6 as a function of disorder increase. (a, b, e, f) Adapted with permission from ref (70), Copyright 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (c) Reprinted with permission from ref (19), Copyright 2018, American Chemical Society. (g) Adapted with permission from ref (67), Copyright 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
The degree of ordering of B-site cations in Cs2AgBiBr6 was investigated with a few different approaches, summarized in Figure 5. Substituting Bi with either In or Sb should cause the band gap to increase or decrease, respectively, in a monotonic fashion. The corresponding simulated band structure, on the other hand, shows contradictory results only for intermediate amounts of In (25%), where the CBM and the band gap were predicted to be slightly lowered instead, see Figure 5e.70 As a result, for heavy amounts of In, or for Sb substitution, the expected trends were otherwise found, leading to the conclusion that the complete Bi/In ordering in the supercell does not reflect the actual structure, and some kind of clustering of the [InBr6] octahedra occurs. Other authors later examined the Ag/Bi distribution in stoichiometric Cs2AgBiBr6, allowing for different local deviations from the perfect alternation. Yang et al. in ref (19), through DFT HSE+SOC calculations, demonstrated that by modifying the ordering parameters, the indirect band gap can vary from 1.93 eV for a fully ordered structure to 0.44 eV for a completely random distribution (see Figure 5c). Recently, Liu et al.108 studied the origin of band gap reduction in disordered Cs2AgBiBr6 using the same level of theory (HSE+SOC). They attributed this reduction to Ag/Bi disorder, which induces different Ag–Br–Ag and Bi–Br–Bi configurations, forming homoatomic clusters that cause wave function localization at the band edges. By analyzing various Ag/Bi alloy ratios, they quantified the degree of disorder and calculated the corresponding band gap reduction, reaching 0.6 eV at 25% disorder. A disorder level of 12.5% has minimal impact on the lattice constants, whereas higher alloy ratios lead to lattice expansion and structural distortion, affecting the stability of Ag-HDP. Experimentally, the degree of ordering can be modified by controlling the growth temperature of the perovskites. Ji et al. developed a crystal-engineering strategy to control the cation alternation in Cs2AgBiBr6.67 By using different evaporation temperatures during crystallization, the band gap was lowered by decreasing the ordering at the Ag–Bi sites. This is evident in the electronic density of states (DOS) calculated at the HSE+SOC level. As the disorder increases, defects are introduced in the Bi-p/Br-p states, progressively decreasing the conduction band, as shown in Figure 5g. While interesting from the point of view of fundamental modeling, it should also be pointed out that such a large segregation would lead to significant and perhaps unrealistic local charge imbalance.
Yang et al. demonstrated that the gap of the perovskite Cs2AgInxBi1–xCl6 can be tuned from indirect (at low concentrations of In, x ≤ 0.25) to direct (at high concentrations of In, x > 0.75).99 This tuning also significantly impacts the photoluminescence quantum efficiency, increasing it by a factor of 5 (Figure 6a). However, DFT/PBE calculations indicate that the emission arises from a forbidden direct transition. Efforts to alter the forbidden direct character of double perovskites are ongoing through alternative alloying strategies. Luo et al. found that incorporating Na into the Cs2AgInCl6 perovskite modifies the parity of the electronic wave function, thereby increasing the probability of electronic transitions. By calculating the transition dipole moment (μ), they observed that the transition probability increases as the Na percentage rises to 40%, and then decreases again at higher concentrations.17 This aligns with their own experimental results showing that Cs2AgInCl6 with a 40% Na concentration exhibits a three-orders-of-magnitude enhancement in photoluminescence. A similar study has been conducted on Bidoped Cs2Ag1–xNaxInCl6 by Locardi et al. They found that photoluminescence was enhanced for Bi and Na concentrations of 5% and 60%, respectively. Through DFT/PBE level calculations for a 3% Bidoped concentration, they analyzed the DOS, showing that Ag and Bi ions in the Na-rich system act as localization centers for electrons and holes at the band edge (middle section of Figure 6b). Analysis of the atomic orbitals for the CBM and VBM shows that the wave functions are delocalized in the pristine perovskite (left and right panels of Figure 6c). However, doping with Ag and Bi causes the wave functions to become localized (middle section of Figure 6c), allowing efficient trapped exciton emission. Similar to the previous work by Juo et al., they found that the oscillator strength, or dipole moment, increased up to a Na percentage of 66% and then decreased (Figure 6d). The results align with the experimental findings, where the photoluminescence quantum yield increased from 6% to 22% up to a Na concentration of 60% and then decreased. Such behavior corresponds to the strong localization of the hole wave function near the Ag center, which disappears for considerable Na concentrations (>66%).109 More recently, a study combining Tight Binding and DFT found similar trends in the transition probability for Cs2AgInCl6 when alloyed with Na.23
Figure 6.
(a) Photoluminescence quantum efficiency as a function of the In content. The nature of the transition shifts from indirect to direct at high concentration of In. (b) Projected density of states for Cs2NaInCl6 (left panel), Cs2AgInCl6 (right panel), and Bi–Ag doped Cs2NaInCl6 (middle panel). Doping with Ag and Na allows the self-trapped exciton transition. (c) CBM and VBM atomic orbitals for pristine and doped perovskite. Doping with Bi–Ag localizes the wave functions. (d) Oscillator strength as a function of Na content for Bi–Ag doped Cs2NaInCl6 perovskite. (a) Reprinted with permission from ref (99), Copyright 2018, American Chemical Society. (b, c, and d) Reprinted with permission from ref (109), Copyright 2019, American Chemical Society.
Other strategies to change the transition character from indirect to direct in double perovskites have been explored theoretically through doping with divalent cations like Sn2+ and Ge2+ in Cs2AgBiCl6, Cs2AgBiBr6,80 and Cs2AgSbCl6.62 By replacing one monovalent and one trivalent cation with two divalent cations, the charge neutrality is maintained, and at a doping concentration of 25% this was predicted to be energetically favorable by using formation energy and decomposition enthalpy calculations at the DFT/HSE+SOC level. The predicted bandgap values, ranging from 0.9 to 2.5 eV (Figure 7), are suitable for photovoltaic applications, while the optical absorption was enhanced by this kind of doping. On the other hand, Fe alloying in Cs2AgBi(Cl,Br)6 perovskites has been studied and synthesized, demonstrating a band gap transition from indirect to direct. Fe incorporation lowers and restructures the CBM, enabling band gap tunability in the range of 1 to 2.5 eV and enhancing the photoluminescence quantum yield. These properties highlight the potential applications of these materials in photoelectronic and photovoltaic devices.74,75 These studies provide valuable guidance for the future electronic structure engineering of double perovskites. Nonetheless, given that photoluminescence in HDP is generally low, further investigations are required to evaluate the transition probabilities of the reported direct gap compounds and explore methods for their improvement. Furthermore, since many of these studies are focused on cubic symmetries, it is crucial to consider potential transitions to more stable nonperovskite phases and the strategies to suppress such transitions in order to enhance stability.
Figure 7.
DFT/HSE+SOC bandgaps for divalent doping in (a) Cs2AgBiBr6, (b) Cs2AgBiCl6, and (c) CsAg2SbCl6. In a)-b), the nature of the transition, Direct or Indirect, is indicated by (D) or (I), respectively. In (c), VAg stands for Ag vacancy. (a and b) Adapted with permission from ref (80), Copyright 2021, American Chemical Society. (c) Adapted with permission of Royal Society of Chemistry, from ref62, Copyright 2022; permission conveyed through Copyright Clearance Center, Inc.
Altogether, bandgap engineering through alloying at the cation and anion sites of double perovskites has been extensively studied using theoretical methods in various perovskite materials. These studies enhance the understanding of the fundamental properties of these materials and offer valuable guidance for experimental syntheses, frequently demonstrating strong agreement. In Table 6, we summarize the band gaps reported for alloying at the monovalent or trivalent cation site of Ag-based HDP. One might expect the position of the bands due to cation doping follow a linear behavior, however, there are several examples where the so-called Bandgap Bowing Effect is observed. In fact, in Table 6, we can see that in most materials and methods, the band gap decreases and then increases again as the value of “x” increases. This nonlinear band gap change in HDP is an open topic, and its causes are still under discussion.88,112 Factors potentially related to this effect include changes in lattice parameters, octahedral distortion, chemical effects that modify the positions of the band states, and ordering. Gupta et al. found a positive correlation between chemical effects, lattice expansion, and bandgap bowing, while octahedral distortion and lattice compression reduce the bowing effect in both Cs2AgxNa1–xInCl6 and Cs2AgInxBi1–xCl6.23
Table 6. Band Gap Values, in Units of eV, for Monovalent and Trivalent Alloying in Ag-Based Perovskitesa.
|
x |
|||||||
|---|---|---|---|---|---|---|---|
| Perovskite | 0.00 | 0.25 | 0.50 | 0.75 | 1.00 | Method | Ref. |
| Cs2(AgxNa1–x)InCl6 | 5.31D | 3.72D | 3.07D | 3.18 D | 3.29D | TB | (23) |
| Cs2(AgxCu1–x)SbCl6 | — | — | 1.8 | 1.88 | 2.22 | HSE | (61) |
| Cs2Ag(InxBi1–x)Cl6 | 2.97 | 2.49 | 2.32 | 2.85D | 3.27D | TB | (23) |
| Cs2Ag(InxBi1–x)Br6 | 2.0 | 1.94 | — | 2.28 D | 1.32 D | HSE* | (70) |
| Cs2Ag(SbxBi1–x)Cl6 | 1.83 | 1.54 | 1.42 | 1.38 | 1.37 | PBE | (107) |
| 1.53 | 1.20 | 1.09 | 1.01 | 1.34 | PBE* | ||
| 3.09 | 2.66 | 2.51 | 2.47 | 2.46 | HSE | ||
| 2.72 | — | 2.10 | — | 2.37 | HSE* | ||
| Cs2Ag(SbxBi1–x)Br6 | 1.77 | 1.18 | 0.94 | 0.82 | 1.37 | HSE* | (88) |
| 2.00 | 1.58 | — | — | 1.67 | HSE* | (70) | |
|
x |
|||||||
|---|---|---|---|---|---|---|---|
| Perovskite | 0.00 | 0.30 | 0.50 | 0.80 | 1.00 | Method | Ref. |
| Cs2Ag(SbxBi1–x)Br6 | 1.32 | 1.26 | 1.14 | 1.04 | 0.89 | PBE | (110) |
| 1.10 | 1.08 | 1.03 | 0.95 | 0.85 | PBE* | ||
| 2.17 | 2.09 | 1.96 | 1.85 | 1.69 | TB09 | ||
| 1.95 | 1.91 | 1.85 | 1.76 | 1.65 | TB09* | ||
Another interesting effect occurs when trivalent cations such as In and Bi are mixed, which give rise to type I (direct) or type II (indirect) materials on their own (Table 6). The indirect character is maintained up to percentages below 75% of the type I cation. When the 75% of the type I cation is exceeded, the delocalized states in the conduction band lower in energy, which changes the nature of the transition from indirect to direct. This effect, also observed experimentally,99 enables the conversion of the indirect transitions to direct through trivalent cation doping, enhancing the photoluminescence quantum yield.
In order to compare the differences between doping at the cation and anion sites, in Table 7 we present the reported band gaps for alloying at the anion site. The bowing effect is absent, and linear behavior is observed in the band gap modification. As “x” increases, the band gap increases linearly. This suppression of the bowing effect may be due to the fact that the ionic radius of Cl (1.81 Å in VI coordination) is slightly smaller than that of Br (1.96 Å), causing the two previously mentioned effects to act as suppressors: (i) there is a lattice parameter compression as the Cl percentage increases and (ii) by alloying the two types of anions, Cl–Br, the octahedra in the material exhibit greater distortion.
Table 7. Band Gap Values for Anion Alloying in Ag-Based Perovskitesa.
| Cs2AgInBr6–xClx |
Cs2AgSbBr6–xClx |
|||||
|---|---|---|---|---|---|---|
| x | (D) Gap (eV) | Method | Ref. | (I) Gap (eV) | Method | Ref. |
| x = 0 | 1.64 | HSE | 98 | 1.68 | HSE | (20) |
| 1.73 | G0W0 | |||||
| 1.77 | PBE0 | (60) | ||||
| x = 1 | 1.92 | HSE | 98 | 1.82 | HSE | (20) |
| 1.90 | G0W0 | |||||
| 1.87 | PBE0 | (60) | ||||
| x = 2 | 2.16 | HSE | 98 | 1.87 | HSE | (20) |
| 1.98 | G0W0 | |||||
| 1.96 | PBE0 | (60) | ||||
| x = 3 | 2.32 | HSE | 98 | 2.01 | HSE | (20) |
| 2.13 | G0W0 | |||||
| 2.22 | PBE0 | (60) | ||||
| x = 4 | 2.43 | HSE | 98 | 2.17 | HSE | (20) |
| 2.28 | G0W0 | |||||
| 2.31 | PBE0 | (60) | ||||
| x = 5 | 2.63 | HSE | 98 | 2.29 | HSE | (20) |
| 2.41 | G0W0 | |||||
| 2.43 | PBE0 | (60) | ||||
| x = 6 | 3.00 | HSE | 98 | 2.34 | HSE | (20) |
| 2.49 | G0W0 | |||||
| 2.72 | PBE0 | (60) | ||||
(D) and (I) stand for direct and indirect gaps, respectively. HSE and G0W0 calculations carried out with VASP, and PBE0 calculations carried out with Crystal17.113
Effect of Pressure
Band gap engineering was observed in double perovskite materials through applied pressure. Pressure was shown to have a direct relationship with changes in excitonic absorption and band gap quenching in cubic perovskites attributed to octahedral contractions and a decrease in atomic bond distances. These changes result in the proximity and overlap of atomic orbitals, causing a closer alignment of the VBM and CBM.3,73,114−116
Li et al. reported
one of the first high-pressure treatment on double perovskites, lowering
the band gap of Cs2AgBiBr6 from 2.2 to 1.7 eV
under pressures up to 15 GPa (see Figure 8a).116 A phase
transition from cubic  to tetragonal (I4/m) was observed. The cubic-tetragonal phase combination
caused a nonlinear change in the band gap over specific pressure ranges,
as shown in Figure 8a. Notably, after releasing the pressure, the band gap is partially
retained at a value of 2.0 eV. Despite the ability to reduce the band
gap, its nature remains indirect and its value is still considerably
high for photovoltaic applications. In line with these findings, Fu
et al. reported on the pressure-induced effects in Cs2AgBiBr6 nanocrystals. They also reported, a phase transition from
cubic to tetragonal at pressures of 2.3 GPa.117 Due to this phase transition, it was noted that as pressure increases,
the band gap first decreases before increasing again. Through DFT
calculations, both cubic and tetragonal phases were studied as functions
of pressure. It was found that the band gap in the cubic phase decreases
as pressure increases (from 0 to 15 GPa), while for the tetragonal
phase, the gap decreases until reaching 3 GPa, after which it begins
to increase (Figure 8b). The evolution of the band gap for both phases is attributed to
modifications in orbital interactions resulting from the tilting and
distortion of the Ag–Br and Bi–Br octahedra under pressure.
These results are consistent with experimental evidence, which indicates
that up to 2.3 GPa, the perovskite phase remains cubic, exhibiting
a decrease in the band gap. Beyond this pressure, a phase transition
occurs from cubic to tetragonal, resulting in an increase in the band
gap.
to tetragonal (I4/m) was observed. The cubic-tetragonal phase combination
caused a nonlinear change in the band gap over specific pressure ranges,
as shown in Figure 8a. Notably, after releasing the pressure, the band gap is partially
retained at a value of 2.0 eV. Despite the ability to reduce the band
gap, its nature remains indirect and its value is still considerably
high for photovoltaic applications. In line with these findings, Fu
et al. reported on the pressure-induced effects in Cs2AgBiBr6 nanocrystals. They also reported, a phase transition from
cubic to tetragonal at pressures of 2.3 GPa.117 Due to this phase transition, it was noted that as pressure increases,
the band gap first decreases before increasing again. Through DFT
calculations, both cubic and tetragonal phases were studied as functions
of pressure. It was found that the band gap in the cubic phase decreases
as pressure increases (from 0 to 15 GPa), while for the tetragonal
phase, the gap decreases until reaching 3 GPa, after which it begins
to increase (Figure 8b). The evolution of the band gap for both phases is attributed to
modifications in orbital interactions resulting from the tilting and
distortion of the Ag–Br and Bi–Br octahedra under pressure.
These results are consistent with experimental evidence, which indicates
that up to 2.3 GPa, the perovskite phase remains cubic, exhibiting
a decrease in the band gap. Beyond this pressure, a phase transition
occurs from cubic to tetragonal, resulting in an increase in the band
gap.
Figure 8.
Pressure-induced modifications of HDP. (a) Experimental band gap of Cs2AgBiBr6 as a function of pressure. The inset provides a zoom on the range from 0 to 4 GPa, where a cubic-to-tetragonal phase transition occurs. The colors differentiate changes in the trends. (b) DFT-calculated band gaps for the cubic and tetragonal phases of Cs2AgBiBr6 as a function of pressure. (a and b) Adapted with permission from ref (116), Copyright 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Several studies have calculated the effects of pressure using DFT for cubic perovskites, including Cs2AgBi(Br,Cl)6,115 Cs2AgSbCl6,73 and Cs2(Ag,Na)In(Bi,Sb)Cl6 alloys.118 A general observation from these studies is that pressure induces a decrease in the band gap. However, as previously described, phase changes may occur, resulting in the opposite trend. Some syntheses of cubic perovskites report considerably lower band gap values compared with typical ones, which are attributed to strain effects. For example, the Cs2AgSbCl6 perovskite has been reported to have the lowest band gap value of 1.82 eV, which is because of pressure effects on its structure.73 DFT calculations confirmed that a reduction in the lattice parameter of the Cs2AgSbCl6 perovskite results in a decrease in the band gap.
So far, we discussed various examples where doping modifies the optical properties of HDP, with these changes attributed to the various electronic states introduced by the dopants. In fact, ionic sizes can also influence properties through strain engineering. Shaek et al. identified shifts in the emission peak of Cs2Ag0.4Na0.6In1–xBixCl6 with varying Bi doping concentrations, while increased Sb concentration in Cs2NaIn1–xSbxCl6 does not result in appreciable changes.118 These behaviors were linked to the electronic properties and ionic sizes of the dopants. While Bi3+ is significantly larger than In3+ , Sb3+ has a very similar ionic radius. The strain induced by Bi substitution indicates that increasing its concentration generates greater strain, resulting in a lower bandgap. Conversely, Sb induces only minor strain, which in turn does not shift the bandgap. DFT calculations reflect an increase in the lattice parameter with higher Bi concentrations, consistent with experimental lattice spacing and the introduction of strain into the system.
Dielectric Function
The optical features of double perovskites determine their possible applications in optoelectronics and/or photovoltaics. These are typically evaluated through the complex dielectric function ε(ω) and the absorption coefficient α(ω). The complex dielectric function
| 11 |
is a fundamental property that defines the interaction of a material with electromagnetic waves. ε(ω) is composed of the real part ε1(ω), which is associated with polarization and refraction, and the imaginary part, ε2(ω), which is related with the possible interband transitions and captures the material’s light absorption. Of particular interest is the static dielectric constant, defined as the real dielectric function at zero frequency, ε1(0). This indicates the polarization response to a static electric field, and is correlated with excitonic binding energy119 and charge mobility. Higher values of ε1(0) correspond to enhanced charge mobility,120 and improved device performances.20,121
The absorption coefficient indicates the amount of light that can be absorbed by the material. It is calculated from the complex dielectric function as follows:
| 12 |
Similarly, other optical properties, such as refractive index η(ω) and reflectivity R(ω), can be derived from the complex dielectric function. Various methods are employed to accurately calculate the dielectric function in double perovskites, including GGA or hybrid functionals, and many-body perturbation theory (MBPT-BSE@GW@PBE).32 The main difference between these methods lies in the estimation of the band edge, leading to different peak positions of the dielectric functions and values for the static dielectric constants. In Figure 9a the absorption, ε2(ω), calculated using different methods for the perovskite Cs2AgSbBr6 is shown. The position of the band edge, the intercept along the energy axis in the plot of the imaginary dielectric function, varies depending on the method used, but the shape of the function exhibits a certain degree of similarity. The static dielectric constant was also calculated, with differences up to 20% depending on the method employed.110 Although the absolute values can vary considerably depending on the calculation approach, most methods show agreement in terms of trends and changes. For example, consistent trends were observed in the increase of the static dielectric constant with increasing values of ‘x’ in Cs2AgSbxBi1–xCl6 across three different theoretical methods.110
Figure 9.
(a) Complex dielectric function of Cs2AgSbBr6 calculated using the PBE, HSE, and BSE-GW-PBE methods. (b) Effects of strain on the real dielectric function of Cs2AgInCl6; different line styles and colors indicate the percentage of strain applied. (a) Adapted with permission from ref (20), Copyright 2024 by the American Physical Society. (b) Reprinted with permission from ref (106), Copyright 2019 Elsevier B.V. All rights reserved.
Interestingly, most theoretical methods agree that Ag-based perovskites exhibit an adequate range of absorption from the calculated dielectric function, primarily within the visible light spectrum and extending into the UV range. Additionally, all calculations predict a high absorption coefficient in Ag-based perovskites, on the order of 105 cm–1, indicating high-intensity optical transitions at the band edge that increase rapidly for higher energy values (refs (20, 40, 48, 80, 96, 102, 106, 107, 110, 122, and 123)). Despite all these favorable predictions, the static dielectric constants of Ag-based perovskites are all relatively low, ranging between 2 and 5, making them less suitable for single-junction solar cells compared with their Pb-based counterparts. However, strategies such as doping or pressure-induced effects can enhance the static dielectric constant. In Figure 9b, it is shown how the real part of the dielectric function is modified by strain, thereby affecting the static dielectric constant. All in all, these materials exhibit a combination of versatility and tunability, making them especially promising for advanced applications, such as photodetectors, visible LEDs, lasers, and UV shielding devices.
Functional Comparison
In this section, we present a highly summarized overview of the functionals used by DFT in the study of HDP properties. There are many other functionals in the literature that can be categorized within the types presented in Table 8.
Table 8. Comparison of DFT functionals for halide double perovskites.
| Functional | Type | Decomposition and Formation Energy | DOS, Bands, and Bandgap | Dielectric Function | Cost |
|---|---|---|---|---|---|
| PBE/PBEsol | GGA | Fair | Underestimated bandgap, | Overestimated | Low |
| May overestimate stability | Good qualitative electronic structure | ||||
| HSE06/ PBE0 | Hybrid | More accurate than PBE | Improved valence, and conduction bands, more accurate gap | More accurate than PBE | High |
| GW | Beyond DFT | (from PBE/HSE06) | Precise electronic structure, gap close to experiment | Best optical description | Very High |
Conclusions
This review gives an outline of the recent significant advancements in the theoretical study of the structural and optical properties of Ag-based HDP through ab initio calculations. DFT has proven essential not only in elucidating the fundamental mechanisms that govern the stability, electronic structure, and optical properties of these materials but also in proposing methods to manipulate and enhance these properties. Ag-based HDP have garnered considerable attention as nontoxic, stable alternatives to lead halide perovskites for optoelectronic applications, and their full potential can be achieved with the help of detailed theoretical and computational analysis that complements experimental research.
To capture the complex behavior of HDP accurately, a variety of DFT software packages have been employed by the scientific community, including VASP, ABINIT, WIEN2k, CASTEP, CRYSTAL, and Quantum Espresso. Each package offers advantages depending on the implementation and optimization of computational methods, system size, and level of precision needed for specific properties. While the input parameters chosen for DFT calculations can vary slightly from one software to another or even within the same software, the general trends show substantial agreement across different packages. In fact, when comparing results, regardless of the software used, the most critical point is the choice of functional. While functionals with the local density approximation (LDA) are the simplest and easiest to implement, they come with significant limitations and limited accuracy. More computationally expensive functionals, with the implementation of the generalized gradient approximation (GGA), such as PBE or PBEsol, reproduce the electronic properties of HDP more accurately but tend to underestimate values such as band gaps or dielectric constants. These functionals are widely used anyway to study thermodynamic stability with reliable results and little computational cost when compared to more precise methods. They also provide an accurate description of the HDP structures, e.g., lattice parameters, electronic density of states, charge densities, and chemical bonding, among others. Hybrid functionals have been used to estimate the electronic properties more accurately but at a higher computational cost. Finally, many-body perturbation theory (in the GW approximation) also improves the accuracy of the electronic and optical predictions, yielding property values that align more closely with experimental measurements. However, also this is computationally expensive and may not be feasible for larger systems or systematic defect analyses. Overall, the choice of functional depends on the target accuracy and available computational resources.
The geometric tolerance factors (i.e., Goldschmidt’s tolerance factor, octahedral factor, and τ factor) provide an indication of how close materials are to the ideal perovskite structure “elpasolite” and, consequently, their overall stability. These factors have served as inputs for theoretical methods, whether DFT, ML, or a combination of both, to narrow down the vast number of materials to those with the most promising optoelectronic properties. Several of these materials were first proposed theoretically and were later successfully synthesized and characterized, as seen in the case of Ag–Bi, In, and Sb HDP. Beyond the first approximation based on tolerance factors, thermodynamic analysis is necessary to determine stability. The formation energy from elements provides a necessary condition of stability during the synthesis; then, the decomposition enthalpy provides a second condition of stability toward decomposition into ternary and binary materials: these latter reactions have direct correlates in the degradation observed often in halide perovskites. General agreement between DFT thermodynamic predictions and experimental synthesis outcomes has been found. Cases such as Cs2AgBiBr6, Cs2AgSbCl6, and alloys of Cs2AgInBr6, Cs2AgSbBr6, and Cs2AgBiCl6 were first predicted based on formation energy calculations and were later successfully synthesized. In turn, decomposition enthalpy calculations have helped us to understand the degradation mechanisms of HDP, as evidenced in the case of Ag HDP into Cs–Ag-halide and Cs-B‴-halide compounds. Iodide Ag-HDP, where both the tolerance factor t and the τ factor indicate a significant deviation from unity, the calculated formation energy is high compared to other HDP, and its decomposition enthalpy reveals highly probable degradation pathways: all of these aspects align with the difficulty in their synthesis. DFT calculations have also been useful for studying defect formation and for proposing experimental strategies to suppress them. In the case of Ag-HDP, one of the most probable defects identified is B‴ vacancies, along with Ag/B‴ antisites. Halogen-B‴ poor or rich environments have been proposed and employed, resulting in the synthesis of more stable HDP. In this way, one can conclude that ab initio thermodynamics calculations provide a deep understanding of the stability of perovskites, not only when evaluating the pure phases but also with respect to defect formation and alloying of different elements.
Theoretical studies have also provided insights into the formation of defects and alloying and into the role of vacancies, dopants, and cation–anion disorder in tuning the optical properties of HDP. DFT calculations show how doping strategies can alter the indirect nature of the optical transitions in HDP, change the parity of the transition to enable direct transitions, and increase the oscillator strength or dipole moment through alloying. This has been experimentally observed through Na doping in Cs2AgInCl6 and In doping in Cs2AgBiCl6, where the transition is modulated from indirect to direct, increasing the quantum efficiency of the perovskites. The degree of disorder of the B-site cations plays a fundamental role in tuning the band gap and the overall stability. DFT calculations estimated band gap variations of up to 1.49 eV for a fully random cation-ordered Cs2AgBiBr6 HDP. This finding motivated the investigation of synthesis techniques, which demonstrated that controlling the vaporization temperature during crystallization modified the degree of cation disorder, achieving band gap reductions of up to 0.26 eV. Additionally, variations in the band gap of the HDP, whether due to the defect strategies, or pressure-induced effects, align with experimental observations, as seen in the case of Cs2AgBiCl6 and Cs2AgBiBr6 perovskites.
In conclusion, DFT methods provide a comprehensive framework for understanding, predicting, and optimizing the stability and electronic and optical properties of Ag-based HDP, offering valuable guidance for experimental efforts. For their part, Ag-based HDP has demonstrated high stability, allowing for the inclusion of defects and doping, which facilitates the manipulation and enhancement of their properties, making them an attractive material with potential for high-performance applications. The refinement of DFT methodologies in exchange-correlation functionals, incorporating machine learning methods, optimizing computational resources through GPU parallelization, and the exploration of Ag-HDP and diverse compositions, particularly through materials and defect engineering, will pave the way for sustainable, nontoxic materials for optoelectronic applications.
Acknowledgments
We acknowledge financial support under the National Recovery and Resilience Plan (NRRP), Mission 4, Component 2, Investment 1.1, Call for tender No. 104 published on 2.2.2022 by the Italian Ministry of University and Research (MUR), funded by the European Union-NextGenerationEU-Project Title Novel SUstainable double PERovskites: multiscale characterization from the atomic structure to functional properties (SUPER)–CUP B53D230150 10006–Grant Assignment Decree No. 1064 adopted on 18.7.2023 by the Italian Ministry of Ministry of University and Research (MUR).
The authors declare no competing financial interest.
References
- Park N.-G.; Grätzel M.; Miyasaka T.; Zhu K.; Emery K. Towards stable and commercially available perovskite solar cells. Nature Energy 2016, 1, 16152. 10.1038/nenergy.2016.152. [DOI] [Google Scholar]
- Fakharuddin A.; Gangishetty M. K.; Abdi-Jalebi M.; Chin S.-H.; bin Mohd Yusoff A. R.; Congreve D. N.; Tress W.; Deschler F.; Vasilopoulou M.; Bolink H. J. Perovskite light-emitting diodes. Nat. Electron. 2022, 5, 203–216. 10.1038/s41928-022-00745-7. [DOI] [Google Scholar]
- Kumar V.; Kathiravan A.; Jhonsi M. A. Beyond Lead Halide Perovskites: Crystal Structure, Bandgaps, Photovoltaic Properties and Future Stance of Lead-Free Halide Double Perovskites. Nano Energy 2024, 125, 109523. 10.1016/j.nanoen.2024.109523. [DOI] [Google Scholar]
- Kim J. Y.; Lee J.-W.; Jung H. S.; Shin H.; Park N.-G. High-efficiency perovskite solar cells. Chem. Rev. 2020, 120, 7867–7918. 10.1021/acs.chemrev.0c00107. [DOI] [PubMed] [Google Scholar]
- Xiao Z.; Song Z.; Yan Y. From lead halide perovskites to lead-free metal halide perovskites and perovskite derivatives. Adv. Mater. 2019, 31, 1803792. 10.1002/adma.201803792. [DOI] [PubMed] [Google Scholar]
- Herz L. M. Charge-carrier mobilities in metal halide perovskites: fundamental mechanisms and limits. ACS Energy Lett. 2017, 2, 1539–1548. 10.1021/acsenergylett.7b00276. [DOI] [Google Scholar]
- Chen C.-H.; Cheng S.-N.; Cheng L.; Wang Z.-K.; Liao L.-S. Toxicity, leakage, and recycling of lead in perovskite photovoltaics. Adv. Energy Mater. 2023, 13, 2204144. 10.1002/aenm.202204144. [DOI] [Google Scholar]
- Wu M.; Haji Ladi N.; Yi Z.; Li H.; Shen Y.; Wang M. Stability Issue of Perovskite Solar Cells under Real-World Operating Conditions. Energy Technology 2020, 8, 1900744. 10.1002/ente.201900744. [DOI] [Google Scholar]
- Yang W.-F.; Igbari F.; Lou Y.-H.; Wang Z.-K.; Liao L.-S. Tin halide perovskites: progress and challenges. Adv. Energy Mater. 2020, 10, 1902584. 10.1002/aenm.201902584. [DOI] [Google Scholar]
- Huang J.; Xiang H.; Ran R.; Zhou W.; Wang W.; Shao Z. Fundamental understanding in the performance-limiting factors of Cs2AgBiBr6-based perovskite photovoltaics. Renewable and Sustainable Energy Reviews 2024, 191, 114187. 10.1016/j.rser.2023.114187. [DOI] [Google Scholar]
- Liu Y.; Nag A.; Manna L.; Xia Z. Lead-free double perovskite Cs2AgInCl6. Angew. Chem. 2021, 133, 11696–11707. 10.1002/ange.202011833. [DOI] [PubMed] [Google Scholar]
- Chand Yadav S.; Srivastava A.; Manjunath V.; Kanwade A.; Devan R. S.; Shirage P. M. Properties, performance and multidimensional applications of stable lead-free Cs2AgBiBr6 double perovskite. Materials Today Physics 2022, 26, 100731. 10.1016/j.mtphys.2022.100731. [DOI] [Google Scholar]
- Lei H.; Hardy D.; Gao F. Lead-free double perovskite Cs2AgBiBr6: fundamentals, applications, and perspectives. Adv. Funct. Mater. 2021, 31, 2105898. 10.1002/adfm.202105898. [DOI] [Google Scholar]
- Wu S.; Liu Y. Recent advancements and manipulation strategies of colloidal Cs2BIBIIIX6 lead-free halide double perovskite nanocrystals. Nano Research 2023, 16, 5572–5591. 10.1007/s12274-022-5232-3. [DOI] [Google Scholar]
- Dahl J. C.; Osowiecki W. T.; Cai Y.; Swabeck J. K.; Bekenstein Y.; Asta M.; Chan E. M.; Alivisatos A. P. Probing the stability and band gaps of Cs2AgInCl6 and Cs2AgSbCl6 lead-free double perovskite nanocrystals. Chem. Mater. 2019, 31, 3134–3143. 10.1021/acs.chemmater.8b04202. [DOI] [Google Scholar]
- Xu J.; Liu J.-B.; Liu B.-X.; Wang J.; Huang B. Defect engineering of grain boundaries in lead-free halide double perovskites for better optoelectronic performance. Adv. Funct. Mater. 2019, 29, 1805870. 10.1002/adfm.201805870. [DOI] [Google Scholar]
- Luo J.; Wang X.; Li S.; Liu J.; Guo Y.; Niu G.; Yao L.; Fu Y.; Gao L.; Dong Q.; et al. Efficient and stable emission of warm-white light from lead-free halide double perovskites. Nature 2018, 563, 541–545. 10.1038/s41586-018-0691-0. [DOI] [PubMed] [Google Scholar]
- Cai Y.; Xie W.; Teng Y. T.; Harikesh P. C.; Ghosh B.; Huck P.; Persson K. A.; Mathews N.; Mhaisalkar S. G.; Sherburne M.; et al. High-throughput computational study of halide double perovskite inorganic compounds. Chem. Mater. 2019, 31, 5392–5401. 10.1021/acs.chemmater.9b00116. [DOI] [Google Scholar]
- Yang J.; Zhang P.; Wei S.-H. Band structure engineering of Cs2AgBiBr6 perovskite through order–disordered transition: A first-principle study. journal of physical chemistry letters 2018, 9, 31–35. 10.1021/acs.jpclett.7b02992. [DOI] [PubMed] [Google Scholar]
- Adhikari S.; Johari P. Capturing optoelectronic properties of Cs2AgSbBr6–xClx (x = 0–6) double perovskites using many-body perturbation theory. Phys. Rev. B 2024, 110, 014101. 10.1103/PhysRevB.110.014101. [DOI] [Google Scholar]
- Xiao Z.; Du K.-Z.; Meng W.; Mitzi D. B.; Yan Y. Chemical origin of the stability difference between copper (I)-and silver (I)-Based halide double perovskites. Angew. Chem. 2017, 129, 12275–12279. 10.1002/ange.201705113. [DOI] [PubMed] [Google Scholar]
- Jöbsis H. J.; Fykouras K.; Reinders J. W.; van Katwijk J.; Dorresteijn J. M.; Arens T.; Vollmer I.; Muscarella L. A.; Leppert L.; Hutter E. M. Conduction band tuning by controlled alloying of Fe into Cs2AgBiBr6 double perovskite powders. Adv. Funct. Mater. 2024, 34, 2306106. 10.1002/adfm.202306106. [DOI] [Google Scholar]
- Gupta M.; Jana S.; Nanda B. Electronic structure and optoelectronic properties of halide double perovskites: Fundamental insights and design of a theoretical workflow. Chem. Mater. 2024, 36, 132–145. 10.1021/acs.chemmater.3c01048. [DOI] [Google Scholar]
- Kresse G.; Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Computational materials science 1996, 6, 15–50. 10.1016/0927-0256(96)00008-0. [DOI] [PubMed] [Google Scholar]
- Gonze X.; Amadon B.; Anglade P.-M.; Beuken J.-M.; Bottin F.; Boulanger P.; Bruneval F.; Caliste D.; Caracas R.; Côté M.; et al. ABINIT: First-principles approach to material and nanosystem properties. Comput. Phys. Commun. 2009, 180, 2582–2615. 10.1016/j.cpc.2009.07.007. [DOI] [Google Scholar]
- Blaha P.; Schwarz K.; Tran F.; Laskowski R.; Madsen G. K.; Marks L. D. WIEN2k: An APW+ lo program for calculating the properties of solids. J. Chem. Phys. 2020, 152, 074101. 10.1063/1.5143061. [DOI] [PubMed] [Google Scholar]
- Segall M.; Lindan P. J.; Probert M. a.; Pickard C. J.; Hasnip P. J.; Clark S.; Payne M. First-principles simulation: ideas, illustrations and the CASTEP code. J. Phys.: Condens. Matter 2002, 14, 2717. 10.1088/0953-8984/14/11/301. [DOI] [Google Scholar]
- Giannozzi P.; Baroni S.; Bonini N.; Calandra M.; Car R.; Cavazzoni C.; Ceresoli D.; Chiarotti G. L.; Cococcioni M.; Dabo I.; et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys.: Condens. Matter 2009, 21, 395502. 10.1088/0953-8984/21/39/395502. [DOI] [PubMed] [Google Scholar]
- Perdew J. P.; Wang Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244. 10.1103/PhysRevB.45.13244. [DOI] [PubMed] [Google Scholar]
- Perdew J. P.; Ruzsinszky A.; Csonka G. I.; Vydrov O. A.; Scuseria G. E.; Constantin L. A.; Zhou X.; Burke K. Restoring the density-gradient expansion for exchange in solids and surfaces. Physical review letters 2008, 100, 136406. 10.1103/PhysRevLett.100.136406. [DOI] [PubMed] [Google Scholar]
- Franchini C. Hybrid functionals applied to perovskites. J. Phys.: Condens. Matter 2014, 26, 253202. 10.1088/0953-8984/26/25/253202. [DOI] [PubMed] [Google Scholar]
- Albrecht S.; Reining L.; Del Sole R.; Onida G. Ab initio calculation of excitonic effects in the optical spectra of semiconductors. Physical review letters 1998, 80, 4510. 10.1103/PhysRevLett.80.4510. [DOI] [Google Scholar]
- Aryasetiawan F.; Gunnarsson O. The GW method. Rep. Prog. Phys. 1998, 61, 237. 10.1088/0034-4885/61/3/002. [DOI] [Google Scholar]
- Pilania G.; Mannodi-Kanakkithodi A.; Uberuaga B.; Ramprasad R.; Gubernatis J.; Lookman T. Machine learning bandgaps of double perovskites. Sci. Rep. 2016, 6, 19375. 10.1038/srep19375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talapatra A.; Uberuaga B. P.; Stanek C. R.; Pilania G. A machine learning approach for the prediction of formability and thermodynamic stability of single and double perovskite oxides. Chem. Mater. 2021, 33, 845–858. 10.1021/acs.chemmater.0c03402. [DOI] [Google Scholar]
- Liu Y.; Cleveland I. J.; Tran M. N.; Aydil E. S. Stability of the halide double perovskite Cs2AgInBr6. J. Phys. Chem. Lett. 2023, 14, 3000–3006. 10.1021/acs.jpclett.3c00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure E. T.; Ball M. R.; Windl W.; Woodward P. M. Cs2AgBiX6 (X= Br, Cl): new visible light absorbing, lead-free halide perovskite semiconductors. Chem. Mater. 2016, 28, 1348–1354. 10.1021/acs.chemmater.5b04231. [DOI] [Google Scholar]
- Rodrigues J. E. F.; Escanhoela Jr C. A.; Fragoso B.; Sombrio G.; Ferrer M. M.; Álvarez-Galván C.; Fernández-Díaz M. T.; Souza J. A.; Ferreira F. F.; Pecharromán C.; et al. Experimental and theoretical investigations on the structural, electronic, and vibrational properties of Cs2AgSbCl6 double perovskite. Ind. Eng. Chem. Res. 2021, 60, 18918–18928. 10.1021/acs.iecr.1c02188. [DOI] [Google Scholar]
- Volonakis G.; Haghighirad A. A.; Milot R. L.; Sio W. H.; Filip M. R.; Wenger B.; Johnston M. B.; Herz L. M.; Snaith H. J.; Giustino F. Cs2InAgCl6: a new lead-free halide double perovskite with direct band gap. journal of physical chemistry letters 2017, 8, 772–778. 10.1021/acs.jpclett.6b02682. [DOI] [PubMed] [Google Scholar]
- Adhikari S.; Johari P. Theoretical insights into monovalent-metal-cation transmutation effects on lead-free halide double perovskites for optoelectronic applications. Phys. Rev. Mater. 2023, 7, 075401. 10.1103/PhysRevMaterials.7.075401. [DOI] [Google Scholar]
- Slavney A. H.; Hu T.; Lindenberg A. M.; Karunadasa H. I. A bismuth-halide double perovskite with long carrier recombination lifetime for photovoltaic applications. Journal of the American chemical society 2016, 138, 2138–2141. 10.1021/jacs.5b13294. [DOI] [PubMed] [Google Scholar]
- Yang B.; Chen J.; Yang S.; Hong F.; Sun L.; Han P.; Pullerits T.; Deng W.; Han K. Lead-free silver-bismuth halide double perovskite nanocrystals. Angew. Chem. 2018, 130, 5457–5461. 10.1002/ange.201800660. [DOI] [PubMed] [Google Scholar]
- Bekenstein Y.; Dahl J. C.; Huang J.; Osowiecki W. T.; Swabeck J. K.; Chan E. M.; Yang P.; Alivisatos A. P. The making and breaking of lead-free double perovskite nanocrystals of cesium silver–bismuth halide compositions. Nano Lett. 2018, 18, 3502–3508. 10.1021/acs.nanolett.8b00560. [DOI] [PubMed] [Google Scholar]
- Gao W.; Ran C.; Xi J.; Jiao B.; Zhang W.; Wu M.; Hou X.; Wu Z. High-quality Cs2AgBiBr6 double perovskite film for lead-free inverted planar heterojunction solar cells with 2.2% efficiency. ChemPhysChem 2018, 19, 1696–1700. 10.1002/cphc.201800346. [DOI] [PubMed] [Google Scholar]
- Greul E.; Petrus M. L.; Binek A.; Docampo P.; Bein T. Highly stable, phase pure Cs2AgBiBr6 double perovskite thin films for optoelectronic applications. Journal of Materials Chemistry A 2017, 5, 19972–19981. 10.1039/C7TA06816F. [DOI] [Google Scholar]
- Bartel C. J.; Sutton C.; Goldsmith B. R.; Ouyang R.; Musgrave C. B.; Ghiringhelli L. M.; Scheffler M. New tolerance factor to predict the stability of perovskite oxides and halides. Science advances 2019, 5, eaav0693 10.1126/sciadv.aav0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Li X.; Zeng H. Lead-free halide double perovskites: structure, luminescence, and applications. Small Structures 2021, 2, 2000071. 10.1002/sstr.202000071. [DOI] [Google Scholar]
- Kale A. J.; Chaurasiya R.; Dixit A. Lead-Free Cs2BB’X6 (B: Ag/Au/Cu, B’: Bi/Sb/Tl, and X: Br/Cl/I) Double Perovskites and Their Potential in Energy Conversion Applications. ACS Appl. Energy Mater. 2022, 5, 10427–10445. 10.1021/acsaem.2c00672. [DOI] [Google Scholar]
- R. Varadwaj P. Cs2AgCrX6 (X = Cl, I) double perovskites: A transition-metal-based semiconducting material series with remarkable optics. Nanomaterials 2020, 10, 973. 10.3390/nano10050973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazim M. Z.; Ishfaq M.; Aldaghfag S. A.; Yaseen M.; Khalid M.; Dahshan A. Lead-free silver-indium based halide double perovskites for energy harvesting applications. J. Phys. Chem. Solids 2024, 185, 111756. 10.1016/j.jpcs.2023.111756. [DOI] [Google Scholar]
- Nabi M.; Padelkar S. S.; Jasieniak J. J.; Simonov A. N.; Alam A. Lead-free magnetic double perovskites for photovoltaic and photocatalysis applications. Physical Review Applied 2024, 21, 014063. 10.1103/PhysRevApplied.21.014063. [DOI] [Google Scholar]
- Wang K.-q.; He Y.; Zhang M.; Shi J.-j.; Cai W.-w. Promising lead-free double-perovskite photovoltaic materials Cs2MM’Br6 (M= Cu, Ag, and Au; M’= Ga, In, Sb, and Bi) with an ideal band gap and high power conversion efficiency. J. Phys. Chem. C 2021, 125, 21160–21168. 10.1021/acs.jpcc.1c05699. [DOI] [Google Scholar]
- Tang T.-Y.; Zhao X.-H.; Wei X.-N.; Hu D.-Y.; Gao L.-K.; Tang Y.-L. Study on electronic, mechanical and optical properties of perovskite Cs2AgGaX6 (X= Cl, Br). Journal of Nanoelectronics and Optoelectronics 2021, 16, 1521–1527. 10.1166/jno.2021.3100. [DOI] [Google Scholar]
- Vishnoi P.; Seshadri R.; Cheetham A. K. Why are double perovskite iodides so rare?. J. Phys. Chem. C 2021, 125, 11756–11764. 10.1021/acs.jpcc.1c02870. [DOI] [Google Scholar]
- Xiao Z.; Meng W.; Wang J.; Yan Y. Thermodynamic stability and defect chemistry of bismuth-based lead-free double perovskites. ChemSusChem 2016, 9, 2628–2633. 10.1002/cssc.201600771. [DOI] [PubMed] [Google Scholar]
- Kent G. T.; Morgan E.; Albanese K. R.; Kallistova A.; Brumberg A.; Kautzsch L.; Wu G.; Vishnoi P.; Seshadri R.; Cheetham A. K. Elusive double perovskite iodides: structural, optical, and magnetic properties. Angew. Chem., Int. Ed. 2023, 62, e202306000 10.1002/anie.202306000. [DOI] [PubMed] [Google Scholar]
- Kupfer C.; Elia J.; Kato M.; Osvet A.; Brabec C. J. Mechanochemical Synthesis of Cesium Titanium Halide Perovskites Cs2TiBr6–xIx (x= 0, 2, 4, 6). Crystal Research and Technology 2023, 58, 2200150. 10.1002/crat.202200150. [DOI] [Google Scholar]
- Filip M. R.; Liu X.; Miglio A.; Hautier G.; Giustino F. Phase diagrams and stability of lead-free halide double perovskites Cs2BB’X6: B= Sb and Bi, B’= Cu, Ag, and Au, and X= Cl, Br, and I. J. Phys. Chem. C 2018, 122, 158–170. 10.1021/acs.jpcc.7b10370. [DOI] [Google Scholar]
- Saeed M.; Haq I. U.; Saleemi A. S.; Rehman S. U.; Haq B. U.; Chaudhry A. R.; Khan I. First-principles prediction of the ground-state crystal structure of double-perovskite halides Cs2AgCrX6 (X= Cl, Br, and I). J. Phys. Chem. Solids 2022, 160, 110302. 10.1016/j.jpcs.2021.110302. [DOI] [Google Scholar]
- Laranjeira J. A.; Azevedo S. A.; La Porta F. A.; Ferrer M. M.; Longo E.; Sambrano J. R. Exploring the Halide Exchange Engineering on Lead-free Cs2AgSbCl6−δBrδ Mixed-Halide Double Perovskite: A DFT Study. J. Phys. Chem. C 2024, 128, 11858. 10.1021/acs.jpcc.4c01990. [DOI] [Google Scholar]
- Yu G.; Xue S.; Yin R.; Wu Q.; Gao T.; Song Y.; Wang R.; Cong W.-Y.; Guan C.; Lu Y.-B. How the Copper Dopant Alters the Geometric and Photoelectronic Properties of the Lead-Free Cs2AgSbCl6 Double Perovskite. Advanced Theory and Simulations 2021, 4, 2100142. 10.1002/adts.202100142. [DOI] [Google Scholar]
- Ibrahim I. A.; Chung C.-Y. Lead-free double perovskites: how divalent cations tune the electronic structure for photovoltaic applications. Journal of Materials Chemistry C 2022, 10, 12276–12285. 10.1039/D2TC02903K. [DOI] [Google Scholar]
- Freysoldt C.; Grabowski B.; Hickel T.; Neugebauer J.; Kresse G.; Janotti A.; Van de Walle C. G. First-principles calculations for point defects in solids. Reviews of modern physics 2014, 86, 253–305. 10.1103/RevModPhys.86.253. [DOI] [Google Scholar]
- Kresse G.; Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Physical review b 1999, 59, 1758. 10.1103/PhysRevB.59.1758. [DOI] [Google Scholar]
- Ernzerhof M.; Scuseria G. E. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. 10.1063/1.478401. [DOI] [PubMed] [Google Scholar]
- Bartel C. J.; Clary J. M.; Sutton C.; Vigil-Fowler D.; Goldsmith B. R.; Holder A. M.; Musgrave C. B. Inorganic halide double perovskites with optoelectronic properties modulated by sublattice mixing. J. Am. Chem. Soc. 2020, 142, 5135–5145. 10.1021/jacs.9b12440. [DOI] [PubMed] [Google Scholar]
- Ji F.; Klarbring J.; Wang F.; Ning W.; Wang L.; Yin C.; Figueroa J. S. M.; Christensen C. K.; Etter M.; Ederth T.; et al. Lead-free halide double perovskite Cs2AgBiBr6 with decreased band gap. Angew. Chem. 2020, 132, 15303–15306. 10.1002/ange.202005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M.; Li G.; Tian W.; Jin S.; Tao X.; Jiang Y.; Pidko E. A.; Li R.; Li C. Understanding the effect of crystalline structural transformation for lead-free inorganic halide perovskites. Adv. Mater. 2020, 32, 2002137. 10.1002/adma.202002137. [DOI] [PubMed] [Google Scholar]
- Yu W.; Zou Y.; Wang H.; Qu B.; Chen Z.; Xiao L. Expanding the Absorption of Double Perovskite Cs2AgBiBr6 to NIR Region. J. Phys. Chem. Lett. 2023, 14, 5310–5317. 10.1021/acs.jpclett.3c00900. [DOI] [PubMed] [Google Scholar]
- Du K.-z.; Meng W.; Wang X.; Yan Y.; Mitzi D. B. Bandgap engineering of lead-free double perovskite Cs2AgBiBr6 through trivalent metal alloying. Angew. Chem., Int. Ed. 2017, 56, 8158–8162. 10.1002/anie.201703970. [DOI] [PubMed] [Google Scholar]
- He F.; Wang Y.; Debnath T.; Döblinger M.; Akkerman Q. A.; Feldmann J.; Dey A. Silver-Nanoclusters and Vacancies Influence the Optical Properties of Spherical Cs2AgBiCl6 Nanocrystals. Advanced Optical Materials 2024, 12, 2302898. 10.1002/adom.202302898. [DOI] [Google Scholar]
- Zhou J.; Rong X.; Molokeev M. S.; Zhang X.; Xia Z. Exploring the transposition effects on the electronic and optical properties of Cs2AgSbCl6 via a combined computational-experimental approach. Journal of Materials Chemistry A 2018, 6, 2346–2352. 10.1039/C7TA10062K. [DOI] [Google Scholar]
- Singh A.; Chaurasiya R.; Bheemaraju A.; Chen J.-S.; Satapathi S. Strain-Induced Band-Edge Modulation in Lead-Free Antimony-Based Double Perovskite for Visible-Light Absorption. ACS Appl. Energy Mater. 2022, 5, 3926–3932. 10.1021/acsaem.1c03598. [DOI] [Google Scholar]
- Yin H.; Xian Y.; Zhang Y.; Chen W.; Wen X.; Rahman N. U.; Long Y.; Jia B.; Fan J.; Li W. An emerging lead-free double-perovskite Cs2AgFeCl6: In single crystal. Adv. Funct. Mater. 2020, 30, 2002225. 10.1002/adfm.202002225. [DOI] [Google Scholar]
- Jöbsis H. J.; Fykouras K.; Reinders J. W.; van Katwijk J.; Dorresteijn J. M.; Arens T.; Vollmer I.; Muscarella L. A.; Leppert L.; Hutter E. M. Conduction band tuning by controlled alloying of Fe into Cs2AgBiBr6 double perovskite powders. Adv. Funct. Mater. 2024, 34, 2306106. 10.1002/adfm.202306106. [DOI] [Google Scholar]
- Zeng Z.; Sun M.; Zhang S.; Zhang H.; Shi X.; Ye S.; Huang B.; Du Y.; Yan C.-H. Rare-earth-based perovskite Cs2AgScCl6: Bi for strong full visible spectrum emission. Adv. Funct. Mater. 2022, 32, 2204780. 10.1002/adfm.202204780. [DOI] [Google Scholar]
- Zhang T.; Cai Z.; Chen S. Chemical trends in the thermodynamic stability and band gaps of 980 halide double perovskites: a high-throughput first-principles study. ACS Appl. Mater. Interfaces 2020, 12, 20680–20690. 10.1021/acsami.0c03622. [DOI] [PubMed] [Google Scholar]
- Liang G.-Q.; Zhang J. A machine learning model for screening thermodynamic stable lead-free halide double perovskites. Comput. Mater. Sci. 2022, 204, 111172. 10.1016/j.commatsci.2021.111172. [DOI] [Google Scholar]
- Li W.; Jacobs R.; Morgan D. Predicting the thermodynamic stability of perovskite oxides using machine learning models. Comput. Mater. Sci. 2018, 150, 454–463. 10.1016/j.commatsci.2018.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X.; Li Z.; Yang J. Efficient direct band gap photovoltaic material predicted via doping double perovskites Cs2AgBiX6 (X = Cl, Br). J. Phys. Chem. C 2021, 125, 10868–10875. 10.1021/acs.jpcc.1c01871. [DOI] [Google Scholar]
- Li T.; Zhao X.; Yang D.; Du M.-H.; Zhang L. Intrinsic defect properties in halide double perovskites for optoelectronic applications. Physical Review Applied 2018, 10, 041001. 10.1103/PhysRevApplied.10.041001. [DOI] [Google Scholar]
- Xu J.; Liu J.-B.; Liu B.-X.; Huang B. Intrinsic defect physics in indium-based lead-free halide double perovskites. journal of physical chemistry letters 2017, 8, 4391–4396. 10.1021/acs.jpclett.7b02008. [DOI] [PubMed] [Google Scholar]
- Meng W.; Wang X.; Xiao Z.; Wang J.; Mitzi D. B.; Yan Y. Parity-forbidden transitions and their impact on the optical absorption properties of lead-free metal halide perovskites and double perovskites. journal of physical chemistry letters 2017, 8, 2999–3007. 10.1021/acs.jpclett.7b01042. [DOI] [PubMed] [Google Scholar]
- Slavney A. H.; Leppert L.; Saldivar Valdes A.; Bartesaghi D.; Savenije T. J.; Neaton J. B.; Karunadasa H. I. Small-band-gap halide double perovskites. Angew. Chem. 2018, 130, 12947–12952. 10.1002/ange.201807421. [DOI] [PubMed] [Google Scholar]
- Savory C. N.; Walsh A.; Scanlon D. O. Can Pb-free halide double perovskites support high-efficiency solar cells?. ACS energy letters 2016, 1, 949–955. 10.1021/acsenergylett.6b00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavney A. H.; Leppert L.; Bartesaghi D.; Gold-Parker A.; Toney M. F.; Savenije T. J.; Neaton J. B.; Karunadasa H. I. Defect-induced band-edge reconstruction of a bismuth-halide double perovskite for visible-light absorption. J. Am. Chem. Soc. 2017, 139, 5015–5018. 10.1021/jacs.7b01629. [DOI] [PubMed] [Google Scholar]
- Wei F.; Deng Z.; Sun S.; Hartono N. T. P.; Seng H. L.; Buonassisi T.; Bristowe P. D.; Cheetham A. K. Enhanced visible light absorption for lead-free double perovskite Cs2AgSbBr6. Chem. Commun. 2019, 55, 3721–3724. 10.1039/C9CC01134J. [DOI] [PubMed] [Google Scholar]
- Li Z.; Kavanagh S. R.; Napari M.; Palgrave R. G.; Abdi-Jalebi M.; Andaji-Garmaroudi Z.; Davies D. W.; Laitinen M.; Julin J.; Isaacs M. A.; et al. Bandgap lowering in mixed alloys of Cs2Ag(SbxBi1–x)Br6 double perovskite thin films. Journal of Materials Chemistry A 2020, 8, 21780–21788. 10.1039/D0TA07145E. [DOI] [Google Scholar]
- Filip M. R.; Hillman S.; Haghighirad A. A.; Snaith H. J.; Giustino F. Band gaps of the lead-free halide double perovskites Cs2BiAgCl6 and Cs2BiAgBr6 from theory and experiment. journal of physical chemistry letters 2016, 7, 2579–2585. 10.1021/acs.jpclett.6b01041. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Sun Q.; Lu Y.; Lu F.; Mu X.; Wei S.-H.; Sui M. Hydrogenated Cs2AgBiBr6 for significantly improved efficiency of lead-free inorganic double perovskite solar cell. Nat. Commun. 2022, 13, 3397. 10.1038/s41467-022-31016-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeev Kumar N.; Radhakrishnan R. Electronic, optical and mechanical properties of lead-free halide double perovskites using first-principles density functional theory. Mater. Lett. 2018, 227, 289–291. 10.1016/j.matlet.2018.05.082. [DOI] [Google Scholar]
- Feng H.-J.; Deng W.; Yang K.; Huang J.; Zeng X. C. Double perovskite Cs2BBiX6 (B= Ag, Cu; X= Br, Cl)/TiO2 heterojunction: an efficient Pb-free perovskite interface for charge extraction. J. Phys. Chem. C 2017, 121, 4471–4480. 10.1021/acs.jpcc.7b00138. [DOI] [Google Scholar]
- Palummo M.; Berrios E.; Varsano D.; Giorgi G. Optical properties of lead-free double perovskites by ab initio excited-state methods. ACS Energy Lett. 2020, 5, 457–463. 10.1021/acsenergylett.9b02593. [DOI] [Google Scholar]
- Appadurai T.; Kashikar R.; Sikarwar P.; Antharjanam S.; Nanda B. R. K.; Chandiran A. K. Manipulation of parity and polarization through structural distortion in light-emitting halide double perovskites. Communications Materials 2021, 2, 68. 10.1038/s43246-021-00172-9. [DOI] [Google Scholar]
- Creutz S. E.; Crites E. N.; De Siena M. C.; Gamelin D. R. Colloidal nanocrystals of lead-free double-perovskite (elpasolite) semiconductors: synthesis and anion exchange to access new materials. Nano Lett. 2018, 18, 1118–1123. 10.1021/acs.nanolett.7b04659. [DOI] [PubMed] [Google Scholar]
- Qaid S. M.H.; ul Ain Q.; Ghaithan H. M.; Mursaleen I.; Ahmed Ali Ahmed A.; Munir J. First-principles investigations on the structural, optoelectronic, mechanical and transport properties of new stable lead-free double perovskites Cs2BB’I6 (B= Ag/Rb, B’= Bi/Ga) halides. Materials Science and Engineering: B 2024, 301, 117176. 10.1016/j.mseb.2024.117176. [DOI] [Google Scholar]
- Zhao X.-G.; Yang J.-H.; Fu Y.; Yang D.; Xu Q.; Yu L.; Wei S.-H.; Zhang L. Design of lead-free inorganic halide perovskites for solar cells via cation-transmutation. J. Am. Chem. Soc. 2017, 139, 2630–2638. 10.1021/jacs.6b09645. [DOI] [PubMed] [Google Scholar]
- Liang Y. Exploring inorganic and nontoxic double perovskites Cs2AgInBr6(1–x)Cl6x from material selection to device design in material genome approach. J. Alloys Compd. 2021, 862, 158575. 10.1016/j.jallcom.2020.158575. [DOI] [Google Scholar]
- Yang B.; Mao X.; Hong F.; Meng W.; Tang Y.; Xia X.; Yang S.; Deng W.; Han K. Lead-free direct band gap double-perovskite nanocrystals with bright dual-color emission. J. Am. Chem. Soc. 2018, 140, 17001–17006. 10.1021/jacs.8b07424. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Xia Z.; Molokeev M. S.; Zhang X.; Peng D.; Liu Q. Composition design, optical gap and stability investigations of lead-free halide double perovskite Cs2AgInCl6. Journal of Materials Chemistry A 2017, 5, 15031–15037. 10.1039/C7TA04690A. [DOI] [Google Scholar]
- Luo J.; Li S.; Wu H.; Zhou Y.; Li Y.; Liu J.; Li J.; Li K.; Yi F.; Niu G.; et al. Cs2AgInCl6 double perovskite single crystals: parity forbidden transitions and their application for sensitive and fast UV photodetectors. Acs Photonics 2018, 5, 398–405. 10.1021/acsphotonics.7b00837. [DOI] [Google Scholar]
- Alla M.; Mishra O. P.; Wakale G. R.; Choudhary E.; Manjunath V.; Hossain M. K.; Rouchdi M.; Fares B. Non-Toxic and Stable Double Perovskite Solar Cells Based on Cs2AgSbX6 Light Harvester: First Principle Calculations-Aided Theoretical Estimation. Advanced Theory and Simulations 2024, 7, 2300605. 10.1002/adts.202300605. [DOI] [Google Scholar]
- Heyd J.; Scuseria G. E. Efficient hybrid density functional calculations in solids: Assessment of the Heyd–Scuseria–Ernzerhof screened Coulomb hybrid functional. J. Chem. Phys. 2004, 121, 1187–1192. 10.1063/1.1760074. [DOI] [PubMed] [Google Scholar]
- Becke A. D.; Johnson E. R. A simple effective potential for exchange. J. Chem. Phys. 2006, 124, 221101. 10.1063/1.2213970. [DOI] [PubMed] [Google Scholar]
- Marini A.; Hogan C.; Grüning M.; Varsano D. Yambo: an ab initio tool for excited state calculations. Comput. Phys. Commun. 2009, 180, 1392–1403. 10.1016/j.cpc.2009.02.003. [DOI] [Google Scholar]
- Soni A.; Bhamu K.; Sahariya J. Investigating effect of strain on electronic and optical properties of lead free double perovskite Cs2AgInCl6 solar cell compound: a first principle calculation. J. Alloys Compd. 2020, 817, 152758. 10.1016/j.jallcom.2019.152758. [DOI] [Google Scholar]
- Chen H.; Ming S.; Li M.; Wang B.; Su J. First-Principles Study on the Structure, Electronic and Optical Properties of Cs2AgSbxBi1–xCl6 Double Perovskites. J. Phys. Chem. C 2021, 125, 11271–11277. 10.1021/acs.jpcc.1c03027. [DOI] [Google Scholar]
- Liu D.; Abzakh B. A.; Kazakova E. A.; Abrameshin D. A.; Troshin P. A.; Long R.; Vasenko A. S. Wave Function Localization Reduces the Bandgap of Disordered Double Perovskite Cs2AgBiBr6. J. Phys. Chem. Lett. 2024, 15, 11268–11274. 10.1021/acs.jpclett.4c02946. [DOI] [PubMed] [Google Scholar]
- Locardi F.; Sartori E.; Buha J.; Zito J.; Prato M.; Pinchetti V.; Zaffalon M. L.; Ferretti M.; Brovelli S.; Infante I.; et al. Emissive Bi-doped double perovskite Cs2Ag1–xNaxInCl6 nanocrystals. ACS Energy Lett. 2019, 4, 1976–1982. 10.1021/acsenergylett.9b01274. [DOI] [Google Scholar]
- Thatribud A.; Rassamesard A. First-principles study on structural, electronic, and optical properties of mixed alloys of Cs2Ag(SbxBi1–x)Br6 double perovskites for optoelectronic applications. Comput. Mater. Sci. 2024, 239, 112964. 10.1016/j.commatsci.2024.112964. [DOI] [Google Scholar]
- Tran F.; Blaha P. Accurate Band Gaps of Semiconductors and Insulators with a Semilocal Exchange-Correlation Potential. Physical review letters 2009, 102, 226401. 10.1103/PhysRevLett.102.226401. [DOI] [PubMed] [Google Scholar]
- Goyal A.; McKechnie S.; Pashov D.; Tumas W.; Van Schilfgaarde M.; Stevanovic V. Origin of pronounced nonlinear band gap behavior in lead–tin hybrid perovskite alloys. Chem. Mater. 2018, 30, 3920–3928. 10.1021/acs.chemmater.8b01695. [DOI] [Google Scholar]
- Dovesi R.; Orlando R.; Civalleri B.; Roetti C.; Saunders V. R.; Zicovich-Wilson C. M. CRYSTAL: a computational tool for the ab initio study of the electronic properties of crystals. Zeitschrift für Kristallographie-Crystalline Materials 2005, 220, 571–573. 10.1524/zkri.220.5.571.65065. [DOI] [Google Scholar]
- Lv C.; Yang X.; Shi Z.; Wang L.; Sui L.; Li Q.; Qin J.; Liu K.; Zhang Z.; Li X.; et al. Pressure-induced ultra-broad-band emission of a Cs2AgBiBr6 perovskite thin film. J. Phys. Chem. C 2020, 124, 1732–1738. 10.1021/acs.jpcc.9b11187. [DOI] [Google Scholar]
- Parrey I. D.; Bilican F.; Kursun C.; Kart H. H.; Parrey K. A. Mechanical stability and energy gap evolution in Cs-based Ag, Bi halide double perovskites under high pressure: A theoretical DFT approach. ACS omega 2023, 8, 26577–26589. 10.1021/acsomega.3c03469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.; Wang Y.; Pan W.; Yang W.; Zou B.; Tang J.; Quan Z. High-pressure band-gap engineering in lead-free Cs2AgBiBr6 double perovskite. Angew. Chem., Int. Ed. 2017, 56, 15969–15973. 10.1002/anie.201708684. [DOI] [PubMed] [Google Scholar]
- Fu R.; Chen Y.; Yong X.; Ma Z.; Wang L.; Lv P.; Lu S.; Xiao G.; Zou B. Pressure-induced structural transition and band gap evolution of double perovskite Cs2AgBiBr6 nanocrystals. Nanoscale 2019, 11, 17004–17009. 10.1039/C9NR07030C. [DOI] [PubMed] [Google Scholar]
- Shaek S.; Khalfin S.; Massasa E. H.; Lang A.; Levy S.; Kortstee L. T.; Shamaev B.; Dror S.; Lifer R.; Shechter R.; et al. How Does Local Strain Affect Stokes Shifts in Halide Double Perovskite Nanocrystals?. Chem. Mater. 2023, 35, 9064–9072. 10.1021/acs.chemmater.3c01771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R.; Nag A. Correlation of dielectric confinement and excitonic binding energy in 2D layered hybrid perovskites using temperature dependent photoluminescence. J. Phys. Chem. C 2020, 124, 16177–16185. 10.1021/acs.jpcc.0c04284. [DOI] [Google Scholar]
- Coropceanu V.; Cornil J.; da Silva Filho D. A.; Olivier Y.; Silbey R.; Brédas J.-L. Charge transport in organic semiconductors. Chem. Rev. 2007, 107, 926–952. 10.1021/cr050140x. [DOI] [PubMed] [Google Scholar]
- Liu X.; Xie B.; Duan C.; Wang Z.; Fan B.; Zhang K.; Lin B.; Colberts F. J.; Ma W.; Janssen R. A.; et al. A high dielectric constant non-fullerene acceptor for efficient bulk-heterojunction organic solar cells. Journal of Materials Chemistry A 2018, 6, 395–403. 10.1039/C7TA10136H. [DOI] [Google Scholar]
- Mukhtar M. W.; Ramzan M.; Rashid M.; Hussain A.; Imran M.; Fahim F.; Neffati R. Ab-initio study of lead-free double Perovskites Cs2AgZBr6 (Z= Bi, Sb) for Solar cells and other renewable energy applications. J. Solid State Chem. 2022, 306, 122781. 10.1016/j.jssc.2021.122781. [DOI] [Google Scholar]
- Zhang K.; Zhang L.; Saravana Karthikeyan S. K. S.; Kong C. Y.; Zhang F.; Guo X.; Dang N. N.; Ramaraj S. G.; Liu X. Structural, electronic, optical, elastic, thermodynamic and thermal transport properties of Cs2AgInCl6 and Cs2AgSbCl6 double perovskite semiconductors using a first-principles study. Phys. Chem. Chem. Phys. 2023, 25, 31848–31868. 10.1039/D3CP03795A. [DOI] [PubMed] [Google Scholar]










