Abstract
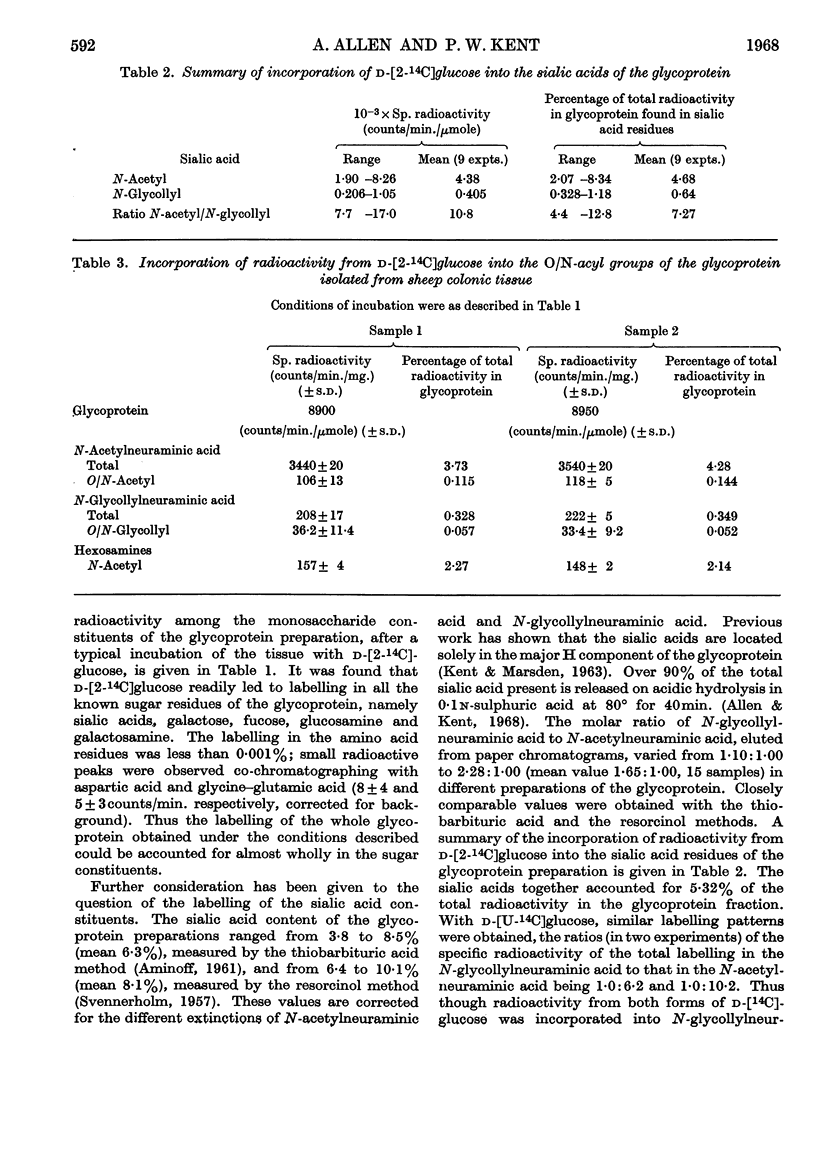
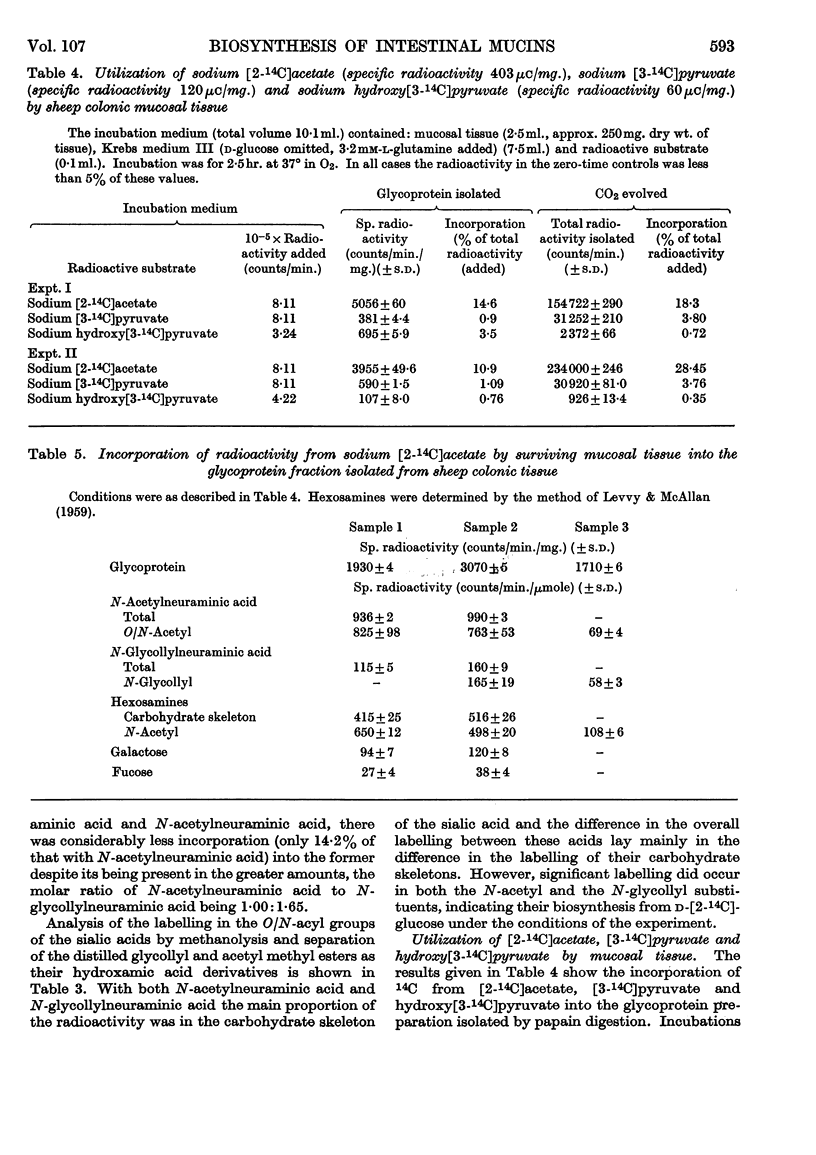
1. d-[2-14C]Glucose, [2-14C]acetate, hydroxy[3-14C]pyruvate, [3-14C]pyruvate and [U-14C]glycine were incorporated by surviving scrapings of sheep colonic mucosal tissue into glycoprotein. 2. d-[2-14C]Glucose, [2-14C]acetate, incorporated hydroxy-[3-14C]pyruvate and [3-14C]pyruvate resulted in labelling of each of the monosaccharide residues of the glycoprotein, namely N-glycollylneuraminic acid, N-acetylneuraminic acid, galactose, fucose, glucosamine and galactosamine. [U-14C]Glycine was incorporated as glycyl and seryl residues of the glycoprotein. 3. Despite N-glycollylneuraminic acid being quantitatively the predominant sialic acid (N-glycollylneuraminic acid and N-acetylneuraminic acid were 8·5 and 5·2% by weight of the glycoprotein respectively) the corresponding ratio of the radio-active labelling from d-[2-14C]glucose in N-glycollylneuraminic acid to that in N-acetylneuraminic acid was 1·00:7·27 (expressed as percentages of the total radioactivity in the glycoprotein). Neutral sugar, hexosamine and N-acetylneuraminic acid residues of the mucoprotein were each labelled to a similar extent. 4. Similarly, the ratio of the radioactivity in N-glycollylneuraminic acid to that in N-acetylneuraminic acid in the mucoprotein from tissue incubations with [2-14C]-acetate was 1·0:4·0. 5. Both [2-14C]acetate and [2-14C]glucose with whole tissue led to labelling of the N-glycollyl substituent and of the main nonose skeleton of the N-glycollylneuraminic acid. In whole-tissue incubations, [3-14C]pyruvate was also a precursor of radioactive N-glycollylneuraminic acid. 6. Hydroxy[3-14C]-pyruvate and [U-14C]glycine caused labelling of the carbohydrate and peptide residues of the glycoprotein, but did not give rise to labelling in the N-glycollylneuraminic acid residues. 7. With a wide variety of possible N-glycollyl precursors (fructose 6-phosphate, hydroxypyruvate, glycollate and chemically synthesized glycollyl-CoA) biosynthesis of N-glycollylglucosamine was not observed in cell-free preparations.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANNISON E. F., LENG R. A., LINDSAY D. B., WHITE R. R. THE METABOLISM OF ACETIC ACID, PROPIONIC ACID AND BUTYRIC ACID IN SHEEP. Biochem J. 1963 Aug;88:248–252. doi: 10.1042/bj0880248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A., Kent P. W. Biosynthesis of intestinal mucins. Effect of puromycin on mucoprotein biosynthesis by sheep colonic mucosal tissue. Biochem J. 1968 Jan;106(1):301–309. doi: 10.1042/bj1060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- CARDINI C. E., LELOIR L. F. Enzymatic formation of acetylgalactosamine. J Biol Chem. 1957 Mar;225(1):317–324. [PubMed] [Google Scholar]
- DAFONSECA-WOLLHEIM F., HEESEN H., HOLZER H. OXYDATIVE DECARBOXYLIERUNG VON HYDROXYPYRUVAT ZU GLYKOLYL-COENZYM A. Biochem Z. 1964 Sep 28;340:383–389. [PubMed] [Google Scholar]
- DATTA A. G., RACKER E. Mechanism of action of transketolase. II. The substrate-enzyme intermediate. J Biol Chem. 1961 Mar;236:624–628. [PubMed] [Google Scholar]
- DAVIDSON E. A., BLUMENTHAL H. J., ROSEMAN S. Glucosamine metabolism. II. Studies on glucosamine 6-phosphate N-acetylase. J Biol Chem. 1957 May;226(1):125–133. [PubMed] [Google Scholar]
- DAWKINS P. D., DICKENS F. THE OXIDATION OF D- AND L-GLYCERATE BY RAT LIVER. Biochem J. 1965 Feb;94:353–367. doi: 10.1042/bj0940353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper P., Kent P. W. Biosynthesis of intestinal mucins. 4. Utilization of [1-C]glucose by sheep colonic mucosa in vitro. Biochem J. 1963 Feb;86(2):248–254. doi: 10.1042/bj0860248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- HOLZER H., KATTERMANN R., BUSCH D. A thiamine pyrophosphate-glycoaldehyde compound ("active glycolaldehyde") as intermediate in the transketolase reaction. Biochem Biophys Res Commun. 1962 Apr 3;7:167–172. doi: 10.1016/0006-291x(62)90168-7. [DOI] [PubMed] [Google Scholar]
- JOURDIAN G. W., ROSEMAN S. Intermediary metabolism of the sialic acids. Ann N Y Acad Sci. 1963 Mar 30;106:202–217. doi: 10.1111/j.1749-6632.1963.tb16639.x. [DOI] [PubMed] [Google Scholar]
- JOURDIAN G. W., ROSEMAN S. The sialic acids. II. Preparation of N-glycolylhexosamines, N-glycolylhexosamine 6-phosphates, glycolyl coenzyme A, and glycolyl glutathione. J Biol Chem. 1962 Aug;237:2442–2446. [PubMed] [Google Scholar]
- Kent P. W., Allen A. The biosynthesis of intestinal mucins. The effect of salicylate on glycoprotein biosynthesis by sheep colonic and human gastric mucosal tissues in vitro. Biochem J. 1968 Feb;106(3):645–658. doi: 10.1042/bj1060645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent P. W., Draper P. Biosynthesis of intestinal mucins. Sialic acids of sheep colonic epithelial mucin. Biochem J. 1968 Jan;106(1):293–299. doi: 10.1042/bj1060293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVVY G. A., MCALLAN A. The N-acetylation and estimation of hexosamines. Biochem J. 1959 Sep;73:127–132. doi: 10.1042/bj0730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUDOWIEG J., DORFMAN A. A micromethod for the colorimetric determination of N-acetyl groups in acid mucopolysaccharides. Biochim Biophys Acta. 1960 Feb 26;38:212–218. doi: 10.1016/0006-3002(60)91233-6. [DOI] [PubMed] [Google Scholar]
- MEISTER A., FRASER P. E., TICE S. V. Enzymatic desulfuration of beta-mercaptopyruvate to pyruvate. J Biol Chem. 1954 Feb;206(2):561–575. [PubMed] [Google Scholar]
- MORETTI A., WOLF G. Vitamin A and mucopolysaccharide biosynthesis in colon homogenates. Biochim Biophys Acta. 1961 Oct 28;53:263–272. doi: 10.1016/0006-3002(61)90439-5. [DOI] [PubMed] [Google Scholar]
- Partridge S. M. Filter-paper partition chromatography of sugars: 1. General description and application to the qualitative analysis of sugars in apple juice, egg white and foetal blood of sheep. with a note by R. G. Westall. Biochem J. 1948;42(2):238–250. doi: 10.1042/bj0420238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- SALLACH H. J. Formation of serine hydroxypryuvate and L-alanine. J Biol Chem. 1956 Dec;223(2):1101–1108. [PubMed] [Google Scholar]
- SENIOR J. R., ISSELBACHER K. J. Activation of long-chain fatty acids by rat-gut mucosa. Biochim Biophys Acta. 1960 Nov 4;44:399–400. doi: 10.1016/0006-3002(60)91594-8. [DOI] [PubMed] [Google Scholar]
- SHERRATT H. S., HUBSCHER G. Properties of mitochondrial preparations from the small-intestinal mucosa of the guinea-pig. Biochim Biophys Acta. 1963 Feb 5;69:403–405. doi: 10.1016/0006-3002(63)91274-5. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- ZELITCH I. THE RELATION OF GLYCOLIC ACID SYNTHESIS TO THE PRIMARY PHOTOSYNTHETIC CARBOXYLATION REACTION IN LEAVES. J Biol Chem. 1965 May;240:1869–1876. [PubMed] [Google Scholar]


