Abstract
Dengue virus replication involves synthesis of a replicative intermediate RNA (RI-RNA), whose presence reveals an actual infection. We report on a simple and rapid reverse transcription-PCR for the detection of viral RI-RNA in infected cells. The product is demonstrated at 20 min postinfection. This method is useful for the study of virus-cell tropism.
Dengue fever and dengue hemorrhagic fever-shock syndrome are caused by four antigenically related single-stranded positive-polarity RNA viruses (DEN-1 to DEN-4) (7). The disease is widely distributed in tropical countries and is transmitted to humans by mosquitoes of the genus Aedes (8). The dengue virus (DV) replication cycle includes translation of the genomic RNA into several proteins, synthesis of negative- and positive-stranded RNAs, and assemblage and release of mature virions (2). Along these processes, three different virus-specific RNA species are required (3, 4). The first type is the positive-stranded genomic RNA, which is translated into a polyprotein; the individual viral proteins are further produced by proteolysis. The second one is a heterogeneous RNA, which is partially RNase resistant and which is named replicative intermediate RNA (RI-RNA). RI-RNA is a complex molecule composed of complementary negative strands and nascent positive single-stranded RNA molecules, whose synthesis is indispensable for the progress of the replicative cycle. The third type is the replicative-form RNA (RF-RNA), which is RNase resistant, is composed of duplex genome-length positive- and negative-stranded RNAs, and can be used as a recycling template for semiconservative replication (1–4).
DV RI-RNA is present in cells only when active replication occurs. Thus, demonstration of such a molecular entity can be used to identify infected cells. This is important since the common procedures used for the identification of infected cells are performed through detection of the viral genome or antigens expressed on the cell surface. The main disadvantage of these methods is their inability to distinguish between intracellular viral synthesis and the passive adherence of molecules to the cell surface (10). Here, we describe a simple and rapid reverse transcription-PCR (RT-PCR) that allows the specific detection of DV RI-RNA. This method could be useful for the study of DV tropism toward host cells during natural infections.
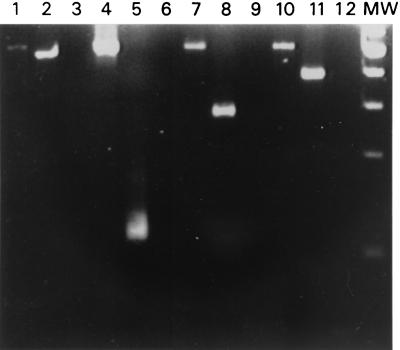
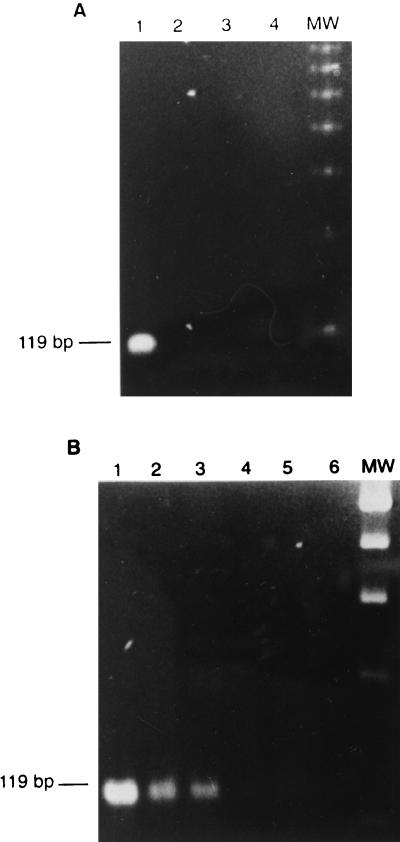
Clinical DEN-1 to DEN-4 isolates were used to detect RI-RNA, as shown in Fig. 1, where it can be seen that the method is equally able to detect any of the serotypes. Viruses were cultured in C6/36 cells at multiplicities of infection (MOIs) of 1 and 10 PFU per cell. RNA was extracted (9) and converted to cDNA with the SuperScript preamplification system (Gibco BRL, Rockville, Md.) with sense primer D1 or antisense type-specific primer TS 1-4, as described by Lanciotti et al. (5) for RI-RNA and genome retrotranscription, respectively. In order to avoid primer annealing and amplification of the viral RF-RNA, the reaction was kept at 42°C to conserve the double-stranded structure. DNA amplification was carried out with the respective sense and antisense primers (primer D1 and corresponding primer TS 1-4 for genome identification or primers D1 and D2 for RI-RNA detection). The samples were subjected to 35 amplification cycles. Each cycle consisted of DNA denaturation for 3 s at 94°C, annealing of primers for 3 s at 57°C, and primer extension for 10 s at 72°C. The expected fragments of 511 bp for the negative strand as well as the corresponding fragments for the positive strands for each DV serotype (482, 119, 290, and 392 bp for DEN-1 to DEN-4, respectively) were observed. For subsequent experiments DEN-2 was used as a model. The intensities of the bands were higher in a manner dependent on the MOI used. The virus stock solution was assessed for the absence of negative-stranded RNA. After RT-PCR, the 511-bp band corresponding to the negative strand was absent, but the characteristic 119-bp band corresponding to DEN-2 was clearly observed (Fig. 2A). The method was able to detect as few as 10 DV particles (Fig. 2B). No bands were seen in the noninfected control.
FIG. 1.
Identification of viral genome and RI-RNA in mosquito C6/36 cells. Cells were infected at an MOI of 10 with DEN-1 (lanes 1 and 2), DEN-2 (lanes 4 and 5), DEN-3 (lanes 7 and 8), and DEN-4 (lanes 10 and 11). Total RNA was subjected to RT-PCR for detection of viral RI-RNA (lanes 1, 4, 7, and 10) and the viral genome (lanes 2, 5, 8, and 11). RNAs from noninfected cells with the corresponding DEN-1- to DEN-4-specific primers were used as negative controls (lanes 3, 6, 9, and 12). Lane MW, molecular size marker (100-bp ladder).
FIG. 2.
Specificity and sensitivity of detection of DV RI-RNA in the viral stock solution by RT-PCR. (A) Stock solutions containing 2 × 107 DEN-2 particles (lanes 1 and 2) and supernatant from noninfected C6/36 cells (lanes 3 and 4) were analyzed by looking for positive (lanes 1 and 3) and negative (lanes 2 and 4) strands. (B) Dilutions from the stock solutions containing 1,000 (lane 1), 100 (lane 2), 10 (lane 3), 1 (lane 4), and 0.1 (lane 5) DEN-2 particles and the supernatant from noninfected C6/36 cells (lane 6) were analyzed by looking for positive strands. Lane MW, molecular size marker (100-bp ladder).
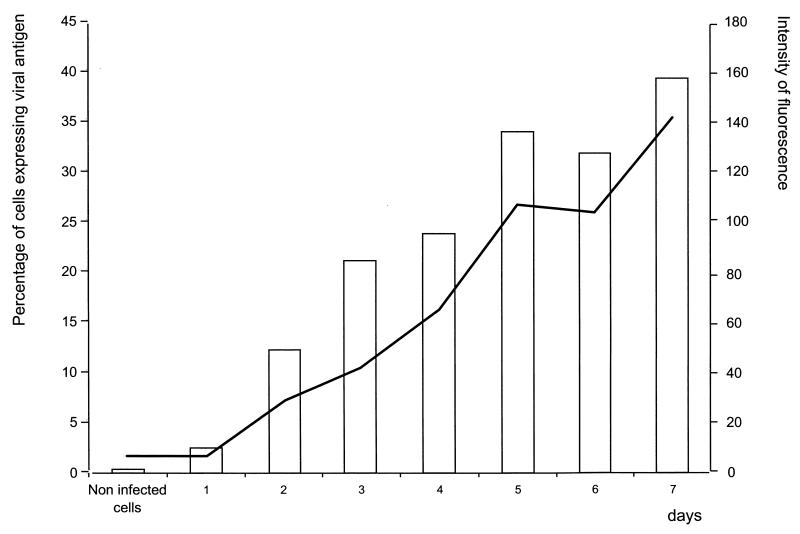
Because detection of the viral envelope (E) protein is conventionally used as a marker of infection, flow cytometry was used to verify the RT-PCR results. Indirect staining of infected and noninfected cells was performed with anti-DEN-2 E protein (clone 3H5; American Type Culture Collection, Manassas, Va.) and fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G. Analysis was carried out in a FACSort cytometer (Becton Dickinson, San Jose, Calif.), and the data were acquired and processed with CellQuest (version 3.3) software. As seen in Fig. 3, positive cells were clearly observed until 72 h postinfection.
FIG. 3.
Detection of viral E protein by flow cytometry. Infected and noninfected C6/36 mosquito cells were harvested and subjected to flow cytometry for detection of the viral E protein on the indicated days postinfection. The bars depict the percentages of antigen-positive cells. The line represents the intensity of fluorescence.
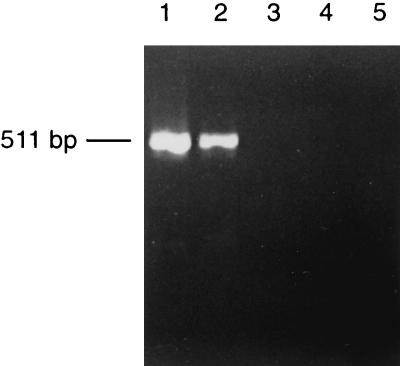
In order to determine the minimum time postinfection required by our method to detect the synthesis of the RNA negative strand, the kinetics of DEN-2 infection in C6/36 cells was determined. Cells were infected at an MOI of 10; harvested at 1, 10, 20, and 30 min postinfection; and submitted to RT-PCR. The negative strand was detected as early as 20 min postinfection, and no amplification was observed at earlier times (Fig. 4).
FIG. 4.
Kinetics of DV RI-RNA synthesis by RT-PCR. C6/36 cells were harvested after 30 (lane 1), 20 (lane 2), 10 (lane 3), and 1 (lane 4) min of infection at an MOI of 10 and were subjected to RT-PCR for detection of DV RNA. Lane 5, noninfected control cells.
The methodology described here displays several important advances regarding simplicity and usefulness. Detection of positive and negative RNA strands is achieved in a simple step with no further procedures needed compared to the method of Liu et al. (6), in which final identification was done by Southern blotting. By the method described here, viral RNA was detected in short periods of time postinfection, and this is attributable to the high sensitivity of the RT-PCR with the primers and the sequences chosen. The denaturation step lasted only 3 s, avoiding prolonged incubation at 94°C and thus extending the half-life of the Taq polymerase.
Furthermore, our procedure allowed the detection of the RI-RNA as early as 20 min postinfection. This is an important improvement in time over methodologies that use E-protein detection. Even though E-protein synthesis and expression occur as early events, demonstration of E-protein synthesis and expression is delayed due to limitations of the available methodology (not before 3 days by flow cytometry).
Detection of RI-RNA as a marker for active viral replication can be a new option for the detection of cells involved in the pathogenesis of dengue in naturally infected humans. It is highly probable that the virus can directly affect cells other than monocytes. However, conventional methods for its identification, like detection of the viral genome or surface antigens, are not able to demonstrate actual active infection because DV could be passively adhered to cell membranes. We are investigating the relevance of RI-RNA detection to understand how different cell types are participating in the pathogenesis of dengue.
Acknowledgments
We acknowledge Dodanim Talavera, Yair Limón, and Claudia de la Cruz for helpful and skillful technical assistance. We also acknowledge Ana Flisser, Vianey Ortíz, and Leopoldo Flores for critical comments on the manuscript.
REFERENCES
- 1.Baltimore, D., and D. Girard. 1996. An intermediate in the synthesis of poliovirus RNA. Proc. Natl. Acad. Sci. USA 56:741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers, T. J., C. S. Hahn, R. Galler, and C. M. Rice. 1990. Flavivirus genome, organization, expression and replication. Annu. Rev. Microbiol. 44:649–688. [DOI] [PubMed] [Google Scholar]
- 3.Chu, P. W. G., and E. G. Westaway. 1985. Replication strategy of Kunjin virus: evidence for recycling role of replicative form RNA as template in semiconservative and asymmetric replication. Virology 140:68–79. [DOI] [PubMed] [Google Scholar]
- 4.Cleaves, G. R., T. E. Ryan, and W. Schlesinger. 1981. Identification and characterization of type 2 dengue virus replicative intermediate and replicative form RNAs. Virology 111:73–83. [DOI] [PubMed] [Google Scholar]
- 5.Lanciotti, R. S., C. H. Calisher, D. J. Gubler, G. J. Chang, and V. Vorndam. 1992. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 30:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu, H. S., Y. L. Lin, and C. C. Chen. 1997. Comparison of various methods of detection of different forms of dengue virus type 2 RNA in cultured cells. Acta Virol. 41:317–324. [PubMed] [Google Scholar]
- 7.Monath, T. P. 1995. Flaviviruses (yellow fever, dengue, dengue hemorrhagic fever, Japanese encephalitis, St Louis encephalitis, tick-borne encephalitis), p.1465–1474. In G. L. Mandell, J. E. Bennett, and R. Dolkin (ed.), Principles and practice of infectious diseases, 4th ed. Churchill Livingstone, New York, N.Y.
- 8.Rice, C. M. 1996. Flaviviridae: the viruses and their replication, p.931–959. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology, 3th ed. Lippincott-Raven Publisher, Philadelphia, Pa.
- 9.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 10.Takeda, S., M. Shibata, T. Morishima, A. Harada, A. Nakao, H. Takagi, and Y. Nagai. 1992. Hepatitis C virus infection in hepatocellular carcinoma. Detection of plus-strand and minus-strand viral RNA. Cancer 70:2255–2259. [DOI] [PubMed] [Google Scholar]