Abstract
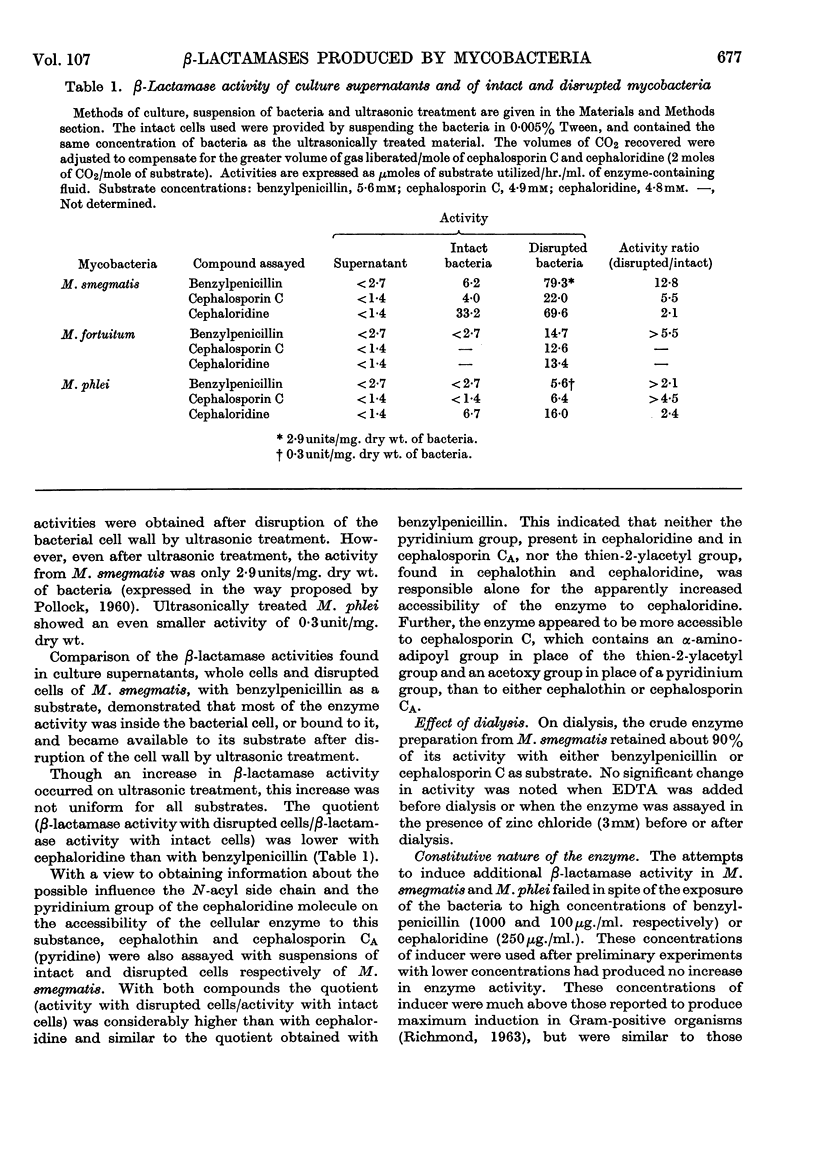
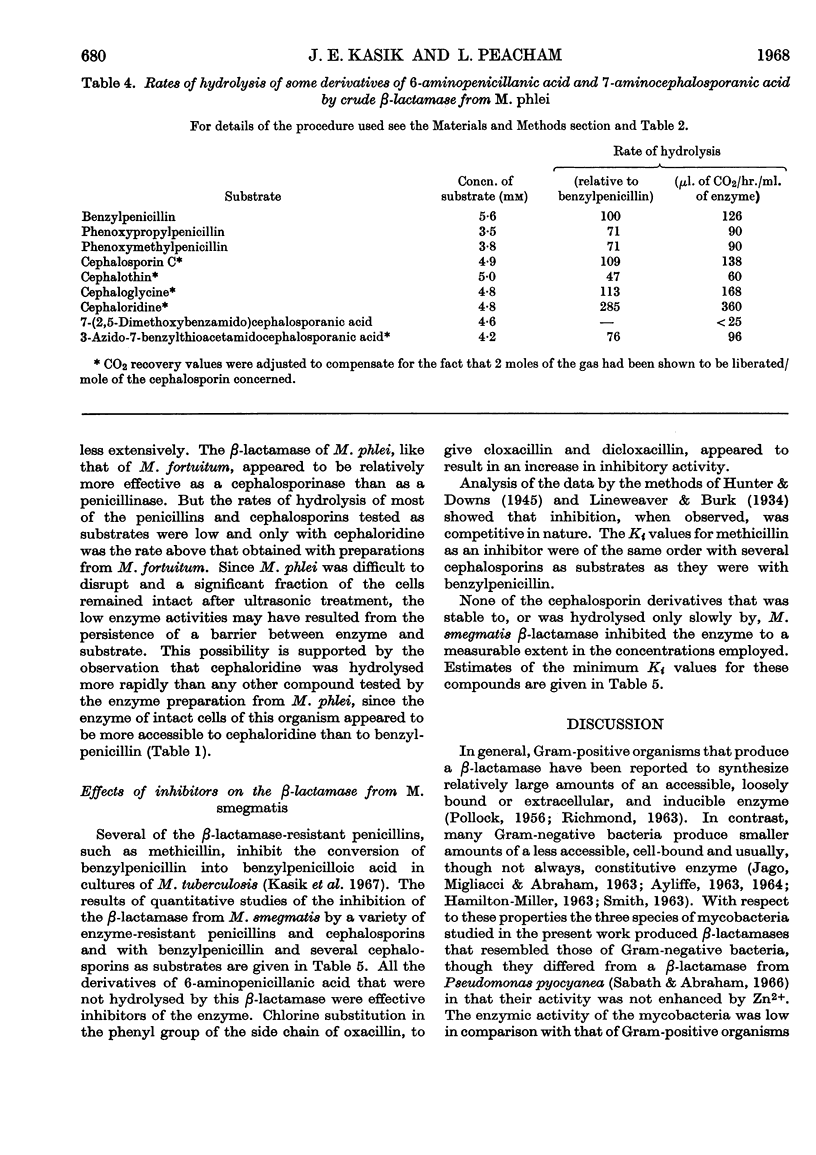
1. Mycobacterium smegmatis (N.C.T.C. 8158), M. fortuitum and M. phlei (MPI) produce a constitutive β-lactamase that has penicillinase and cephalosporinase activity. 2. The β-lactamases of these three species of acid-fast bacteria were mainly cell-bound, only small amounts of activity being liberated into the extracellular fluid. The total β-lactamase activity of these mycobacteria was much lower than that of certain Gram-positive organisms, but comparable with that reported for species of Gram-negative bacteria. 3. The β-lactamases of intact cells of the mycobacteria were not freely accessible to any of the substrates tested, but the apparent crypticity factor to benzylpenicillin was greater than that to cephaloridine and cephalosporin C. 4. Attempts to induce β-lactamase activity in M. smegmatis and M. phlei failed even with high concentrations of inducer. 5. The β-lactamases obtained from the three species of mycobacteria showed different substrate specificities, including different relative activities as cephalosporinases and penicillinases respectively. 6. Certain derivatives of 6-aminopenicillanic acid and 7-aminocephalosporanic acid were found to be resistant to hydrolysis by β-lactamases of M. smegmatis and M. fortuitum. 7. The β-lactamase of M. smegmatis was competitively inhibited by a number of β-lactamase-resistant derivatives of 6-aminopenicillanic acid, but not by similar derivatives of 7-aminocephalosporanic acid.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYLIFFE G. A. Ampicillin inactivation and sensitivity of coliform bacilli. J Gen Microbiol. 1963 Feb;30:339–348. doi: 10.1099/00221287-30-2-339. [DOI] [PubMed] [Google Scholar]
- AYLIFFE G. A. INDUCTION OF CEPHALOSPORINASE AND PENICILLINASE IN PROTEUS SPECIES. Nature. 1964 Mar 7;201:1032–1032. doi: 10.1038/2011032a0. [DOI] [PubMed] [Google Scholar]
- Ayliffe G. A. Cephalosporinase and penicillinase activity of Gram-negative bacteria. J Gen Microbiol. 1965 Jul;40(1):119–126. doi: 10.1099/00221287-40-1-119. [DOI] [PubMed] [Google Scholar]
- CORPE R. F., RUNYON E. H., LESTER W. Status of disease due to unclassified mycobacteria. A statement of the Subcommittee on Unclassified Mycobacteria of the Committee on Therapy. Am Rev Respir Dis. 1963 Mar;87:459–461. doi: 10.1164/arrd.1963.87.3P1.459. [DOI] [PubMed] [Google Scholar]
- CROMPTON B., JAGO M., CRAWFORD K., NEWTON G. G., ABRAHAM E. P. Behaviour of some derivatives of 7-aminocephalosporanic acid and 6-aminopenicillanic acidas substrates, inhibitors and inducers of penicillinases. Biochem J. 1962 Apr;83:52–63. doi: 10.1042/bj0830052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri N., Pollock M. R. The biochemistry and function of beta-lactamase (penicillinase). Adv Enzymol Relat Areas Mol Biol. 1966;28:237–323. doi: 10.1002/9780470122730.ch4. [DOI] [PubMed] [Google Scholar]
- HAMILTON-MILLER J. M. Penicillinase from Klebsiella aerogenes. A comparison with penicillinases from gram-positive species. Biochem J. 1963 Apr;87:209–214. doi: 10.1042/bj0870209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON-MILLER J. M., SMITH J. T. INHIBITION OF PENICILLINASES FROM GRAM-POSITIVE AND GRAM-NEGATIVE BACTERIA BY SUBSTRATE ANALOGUES. Nature. 1964 Mar 7;201:999–1001. doi: 10.1038/201999a0. [DOI] [PubMed] [Google Scholar]
- HAMILTON-MILLER J. M., SMITH J. T., KNOX R. POTENTIATION OF PENICILLIN ACTION BY INHIBITION OF PENICILLINASE. Nature. 1964 Feb 29;201:867–868. doi: 10.1038/201867a0. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Damaging effects of ethylenediaminetetra-acetate and penicillins on permeability barriers in Gram-negative bacteria. Biochem J. 1966 Sep;100(3):675–682. doi: 10.1042/bj1000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Modes of resistance to benzylpenicillin and ampicillin in twelve Klebsiella strains. J Gen Microbiol. 1965 Nov;41(2):175–184. doi: 10.1099/00221287-41-2-175. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M., Smith J. T., Knox R. Interaction of cephaloridine with penicillinase-producing gram-negative bacteria. Nature. 1965 Oct 16;208(5007):235–237. doi: 10.1038/208235a0. [DOI] [PubMed] [Google Scholar]
- JAGO M., MIGLIACCI A., ABRAHAM E. P. PRODUCTION OF A CEPHALOSPORINASE BY PSEUDOMONAS PYOCYANEA. Nature. 1963 Jul 27;199:375–375. doi: 10.1038/199375a0. [DOI] [PubMed] [Google Scholar]
- KASIK J. E. THE NATURE OF MYCOBACTERIAL PENICILLINASE. Am Rev Respir Dis. 1965 Jan;91:117–119. doi: 10.1164/arrd.1965.91.1.117. [DOI] [PubMed] [Google Scholar]
- KOGUT M., POLLOCK M. R., TRIDGELL E. J. Purification of penicillin-induced penicillinase of Bacillus cereus NRRL 569: a comparison of its properties with those of a similarly purified penicillinase produced spontaneously by a constitutive mutant strain. Biochem J. 1956 Mar;62(3):391–401. doi: 10.1042/bj0620391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasik J. E., Weber M., Winberg E., Barclay W. R. The synergistic effect of dicloxacillin and penicillin G on murine tuberculosis. Am Rev Respir Dis. 1966 Aug;94(2):260–261. doi: 10.1164/arrd.1966.94.2.260. [DOI] [PubMed] [Google Scholar]
- PERCIVAL A., BRUMFITT W., DE LOUVOIS J. THE ROLE OF PENICILLINASE IN DETERMINING NATURAL AND ACQUIRED RESISTANCE OF GRAM-NEGATIVE BACTERIA TO PENICILLINS. J Gen Microbiol. 1963 Jul;32:77–89. doi: 10.1099/00221287-32-1-77. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R., RICHMOND M. H. Low cyst(e)ine content of bacterial extracellular proteins: its possible physiological significance. Nature. 1962 May 5;194:446–449. doi: 10.1038/194446a0. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. The cell-bound penicillinase of Bacillus cereus. J Gen Microbiol. 1956 Aug;15(1):154–169. doi: 10.1099/00221287-15-1-154. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. PURIFICATION AND PROPERTIES OF THE EXOPENICILLINASE FROM STAPHYLOCOCCUS AUREUS. Biochem J. 1963 Sep;88:452–459. doi: 10.1042/bj0880452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH J. T. Penicillinase and ampicillin resistance in a strain of Escherichia coli. J Gen Microbiol. 1963 Feb;30:299–306. doi: 10.1099/00221287-30-2-299. [DOI] [PubMed] [Google Scholar]
- SOLTYS M. A. The effect of penicillin on Mycobacteria in vitro and in vivo. Tubercle. 1952 Apr;33(4):120–125. doi: 10.1016/s0041-3879(52)80054-6. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Jago M., Abraham E. P. Cephalosporinase and penicillinase activities of a beta-lactamase from Pseudomonas pyocyanea. Biochem J. 1965 Sep;96(3):739–752. doi: 10.1042/bj0960739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff H. B., Foster J. W. Microbiological Aspects of Penicillin: VII. Bacterial Penicillinase. J Bacteriol. 1945 Jan;49(1):7–17. doi: 10.1128/jb.49.1.7-17.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]


