Abstract
High levels of antileishmanial immunoglobulin E (IgE) antibodies are associated with disease activity in visceral leishmaniasis. Herein, we report our observations about the relationship between antileishmanial IgE antibodies and clinical aspects of cutaneous leishmaniasis. This study was carried out with 45 patients (29 male and 16 female), with ages ranging from 11 to 48 years. All subjects were from an area to which leishmaniasis is endemic, Corte de Pedra (Bahia, Brazil), and the duration of the illness was ≤30 days. The patients were classified as positive or negative for IgE serology in enzyme-linked immunosorbent assay with leishmanial antigens. IgE antibodies were detected in 18 patients (optical density, 0.421 ± 0.30; 95% confidence interval, 0.27 to 0.57), and only 3 (17%) had more than one ulcer. In this group the diameter of Montenegro’s reaction was 18 ± 12.2 mm. In the group with negative IgE serology, 11 of 27 patients (48%) presented two or more cutaneous ulcers, and the mean of the skin test result was 9 ± 6.9 mm. There was a positive correlation between IgE antibody levels and Montenegro’s reaction size and an inverse correlation between IgE antileishmanial antibodies and the number of skin ulcers. The presence of antileishmanial IgE antibodies in cutaneous leishmaniasis may be a result of immunoregulatory events with clinical implications.
Leishmaniasis encompasses diseases resulting from an infection by a protozoan of the Leishmania genus, which presents different clinical forms related to both parasite species and host immune response (8, 20). In an experimental murine model, resistance to the infection by the protozoan depends on the Th1-type immune response, documented by gamma interferon (IFN-γ) production, while susceptibility is related to the Th2 immune response, characterized mainly by interleukin 4 (IL-4), IL-10, and IL-13 specific induction (13, 15).
Human visceral leishmaniasis (VL) has been well characterized by a Th2 immune pattern, demonstrated by significant depression in cellular immunity, failure to produce such proinflammatory cytokines as IFN-γ and IL-2, increased IL-4, IL-5, and IL-10 production, polyclonal B-cell activation, and hypergammaglobulinemia (4, 5, 7, 12, 14, 29). More recently, levels of serum immunoglobulin E (IgE) and antileishmanial IgE antibodies have been documented in VL. Additionally, antileishmanial IgE antibodies are serum markers of disease activity, since they are not documented in individuals with subclinical infection and their titers fall after effective treatment with antimonial drugs (2).
Human cutaneous leishmaniasis (CL) caused in South American by L. braziliensis is characterized by the presence of one or multiple skin ulcers. Unlike the case with VL, patients with CL have a strong Th1 immune response, evidenced by a positive type IV hypersensitivity skin reaction and high IFN-γ production by peripheral blood mononuclear cells stimulated ex vivo by leishmanial antigens (6, 9). Nevertheless, evidence of a Th2 immune response has been reported with this disease, as high levels of serum IgE and mRNA for IL-4, IL-5, and IL-10 in skin biopsies of leishmanial ulcers (3, 11, 19, 21, 23). In this work we evaluated the occurrence of IgE antibodies to leishmanial antigens in sera from CL patients and looked for a relationship between the presence of this isotype of antibody and the following tparameters: clinical features, positivity in skin Montenegro test, and therapeutic response to conventional chemotherapy with a pentavalent antimonial.
MATERIALS AND METHODS
Patients.
Participants of this study (n = 45) were recruited from the area of endemicity of Corte de Pedra located in the state of Bahia, Brazil’s northeastern coast. The diagnosis criteria included the presence of a typical leishmanial skin ulcer and one of the following: detection of the protozoan in culture or in histological slides or a positive leishmania intradermal skin test. In all cases, the illness duration was equal to or less than 30 days and there was no previous history of leishmania infection or previous use of antimonial therapy. Cure was defined as complete cicatrization of the skin ulcer at day 90 after therapy.
Collection of sera.
Sera samples were obtained from blood collected before pentavalent antimonial chemotherapy and immediately after clinical cure. Patients for whom the antimonial therapy failed had blood drawn at day 90 or at 6 months.
Leishmania antigen.
Promastigote forms of L. amazonensis grown in liquid liver infusion tryptose medium, supplemented with 10% fetal calf serum and antibiotics as usual, were thrice washed with phosphate-buffered saline (pH 7.4) and disrupted with 6 mM 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane- sulfonate in 50 mM Tris buffer containing 150 mM NaCl and protease inhibitors (phenylmethylsulfonyl fluoride, leupeptin, tosylsulfonyl phenylalanyl chloromethyl ketone, and pepstatin). The supernatant obtained after centrifugation of the lysate at 5,000 × g was used as the leishmanial antigen source.
IgE antileishmanial antibodies.
Enzyme-linked immunosorbent assay (ELISA) tests for serum IgE antileishmanial antibodies were carried out in wells of micro-ELISA Maxisorp plates (Nunc, Roskilder, Denmark) sensitized with 100 μl of phosphate-buffered saline containing 500 ng of lysate protein, overnight at 4°C. After the wells were washed with Tris-buffered saline (20 mM Tris-HCl, 500 mM NaCl [pH 7.5]) (TBS), their unbound sites were blocked with 1% bovine serum albumin in TBS (TBS-bovine serum albumin [BSA]) at 37°C for 1 h. The plates were subsequently washed with TBS and deionized water, and after drying at 37°C they were used in the ELISA. In order to detect IgE antibodies, 100 μl of the sera diluted 1/6 in TBS-BSA were incubated in the wells for 22 h at 4°C. The wells were thrice washed with TBS containing 0.05% Tween 20 (TBS-T) and submitted to a new incubation with 100 μl of goat anti-human IgE alkaline phosphatase conjugate (Sigma Chemical Co., St. Louis, Mo.) diluted 1/2,500 in TBS-BSA, for 1 h at 22 to 25°C. Next, the plates were washed again with TBS-T, and the reactions were revealed with 100 μl of p-nitrophenylphosphate solution in diethanolamine buffer as usual, for 30 min at room temperature. The absorbances of the products of the enzymatic reactions in the wells were determined at 405 to 600 nm after blockage with 25 μl of 0.5 N NaOH, using a DIAMEDIX Microassay BP 12 apparatus. The cutoff of this IgE ELISA, corresponding to the absorbance of 0.083, was determined through the statistical method of Frey et al. (10), using 10 sera from healthy individuals and 20 sera from persons suffering from Chagas’ disease (n = 5), schistosomiasis (n = 5), atopy (n = 5), and acute toxoplasmosis (n = 5).
Western immunoblotting.
The identification of Leishmania antigens recognized by IgE antibodies was performed through Western immunoblotting (26), using 0.5-cm-width polyvinylidene difluoride (Immobilon; Millipore) strips containing parasite proteins which were initially fractionated by molecular weight on a sodium dodecyl sulfate-12% polyacrylamide gel (17). The immune reactions 2were developed by incubating the strips with sera from CL patients and healthy controls, diluted at 1/10 in TBS-BSA, for 22 h at 4°C. After being washed with TBS-T, the strips were incubated with goat anti-human IgE alkaline phosphatase conjugate diluted at 1/2,500 in TBS-BSA for 1 h at room temperature. Next, the strips were submitted to another wash with TBS-T, and then the immune blots were revealed as purple-colored bands after the membranes were treated with 100 mM Tris-HCl buffer (pH 9.5) containing 5 mM magnesium chloride and 5-bromo-4-chloro-3-indolyl phosphate-nitroblue tetrazolium substrate of alkaline phosphatase.
Statistic analysis.
The statistic Prism PC program (version 1.00; GraphPad Software Inc., 1994) and the chi-square and Mann-Whitney U tests were used to analyze the data.
RESULTS
Clinical features.
The clinical profile of the 45 CL patients is shown in Table 1. The ages ranged from 11 to 48 years ( = 24 ± 10.2), and there was a predominance of male gender (n = 29) (64%). The duration of illness ranged from 8 to 30 days, with a mean of 25 ± 7 days. One ulcerated lesion was documented in 29 (64%) of 45 CL patients, two skin ulcers were observed in 13 (29%), and 3 ulcers were registered in three (7%) cases. The diameters of these ulcers varied from 7.5 to 43.5 mm (x = 17 ± 7.0 mm). The leishmanial skin test was positive in 34 (76%) of 45 CL patients, presenting the reactions mean diameter of 16 ± 9.0 mm.
= 24 ± 10.2), and there was a predominance of male gender (n = 29) (64%). The duration of illness ranged from 8 to 30 days, with a mean of 25 ± 7 days. One ulcerated lesion was documented in 29 (64%) of 45 CL patients, two skin ulcers were observed in 13 (29%), and 3 ulcers were registered in three (7%) cases. The diameters of these ulcers varied from 7.5 to 43.5 mm (x = 17 ± 7.0 mm). The leishmanial skin test was positive in 34 (76%) of 45 CL patients, presenting the reactions mean diameter of 16 ± 9.0 mm.
TABLE 1.
Clinical profile of the 45 CL patients
| Characteristic or test | Ratio or mean ± SD | Variation |
|---|---|---|
| Age (yr) | 24 ± 10.2 | 11–48 |
| Sex (M:F)a | 29:16 | |
| Disease duration (days) | 25 ± 7 | 8–30 |
| Number of lesions (mm) | 1 ± 0.6 | 1–3 |
| Lesion size (mm) | 17 ± 7.0 | 7.5–43.5 |
| Skin test of Montenegro (induration area in mm) | 16 ± 9.0 | 0–50 |
No. of males (M) and females (F).
IgE antileishmanial antibodies.
Positive ELISA IgE tests were verified for 18 (40%) of 45 untreated CL patients, presenting a mean absorbance of 0.421 ± 0.300 (CI [95%], 0.271 to 0.570), as shown in Fig. 1. The treatment of these patients with a pentavalent antimonial was followed by a negativation of IgE antileishmanial antibodies in all but four cases (CI [95%], 0.016 to 0.066) (Fig. 2). The fall of the IgE titer was not related to the cure of the disease, since there was a decrease in the absorbances even in patients that failed therapy. In four cases where the IgE titer remained positive after therapy, three had a complete cure and one failed. On the other hand, for 27 untreated CL patients who were seronegative for IgE antileishmanial antibodies before therapy, the serological immune state did not change after antimonial use. An inverse relationship was observed between the presence of serum IgE antileishmanial antibodies and the number of skin ulcers in CL patients. The size of the skin test, measured in diameter of reaction, was greater in CL patients positive for IgE antileishmanial antibodies (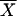 = 18 ± 12.2 mm) than in CL patients seronegative in ELISA for this isotype of antibody (
= 18 ± 12.2 mm) than in CL patients seronegative in ELISA for this isotype of antibody ( = 9 ± 6.9 mm) (Table 2). Additionally, the skin test reactivity was positively correlated with IgE antileishmanial antibody levels detected in these CL patients. The presence of serum IgE antileishmanial antibodies did not anticipate the cure after antimonial treatment, since the percentages of therapeutic failure at day 90 were similar in the two groups, with negative or positive results for IgE antileishmanial antibodies (Table 2). Western immunoblotting antigenic analysis indicated that some bands of molecular mass from 18.5 to 75 kDa, more frequently about 60 kDa, can be recognized by IgE antileishmanial antibodies in sera from untreated CL patients which were positive in ELISA tests.
= 9 ± 6.9 mm) (Table 2). Additionally, the skin test reactivity was positively correlated with IgE antileishmanial antibody levels detected in these CL patients. The presence of serum IgE antileishmanial antibodies did not anticipate the cure after antimonial treatment, since the percentages of therapeutic failure at day 90 were similar in the two groups, with negative or positive results for IgE antileishmanial antibodies (Table 2). Western immunoblotting antigenic analysis indicated that some bands of molecular mass from 18.5 to 75 kDa, more frequently about 60 kDa, can be recognized by IgE antileishmanial antibodies in sera from untreated CL patients which were positive in ELISA tests.
FIG. 1.
Levels of antileishmanial IgE antibodies detected by ELISA in sera of 45 cutaneous leishmaniasis patients before treatment. Specific IgE antibodies were detected in 40% (18 of 45) of these patients, with a mean optical density of 0.180 ± 0.273 (CI [95%], 0.098 to 0.262). The mean value is represented in each column as a drawn line.
FIG. 2.
Levels of antileishmanial IgE antibodies in 18 CL patients before treatment (1) and after treatment (2). OD, optical density.
TABLE 2.
Association between IgE antibodies and clinical aspects of CL
| Presence of antileishmanial IgE antibodies | No. of patients/total (%)
|
Result (mm), mean ± SD
|
||
|---|---|---|---|---|
| Cure at day 90 | Presence of one lesion | Lesion size | Skin test | |
| Positive | 5/18 (28) | 15/18 (83)a | 18 ± 5.4 | 18 ± 12.2b |
| Negative | 10/26 (38) | 14/27 (52) | 17 ± 7.9 | 9 ± 6.9 |
χ20.05 significant level.
Mann-Whitney U test significant level (0.05).
DISCUSSION
The early events of leishmania infection are important in determining control or evolution of the disease. It is well known that the cure of leishmaniasis depends on the appropriate activation of the parasitized macrophages by IFN-γ secreted by Th1 lymphocytes, under the influence of IL-12 and IL-18 (27). When the protozoan encounters a favorable environment for producing Th2 cytokines, such as IL-4, IL-10 and IL-13, which are natural antagonists of the Th1 immune response, the multiplication of leishmania is enhanced and infection evolves into the disease (15, 16, 18, 27). IgE antibodies are produced in the Th2 immune response and have been used in serodiagnosis and also as indicators of the disease activity in toxoplasmosis, cerebral malaria, and VL (2, 22, 28).
CL is a clinical form of the leishmanial infection characterized by an evident Th1 type of immune response, documented by lymphocyte proliferation and IFN-γ secretion. However, high levels of parasitic-specific serum IgE without B-cell polyclonal activation or hypergammaglobulinemia are also observed (21). In this work, we documented the occurrence of IgE antileishmanial antibodies in serum for 40% of the patients with early CL which can react with proteins with molecular masses ranging from 18.5 to 75 kDa. Unlike the case with VL, this isotype of antibody was not related to the activity of the disease or unresponsiveness to pentavalent antimonial chemotherapy (1, 2). In spite of this, there was an inverse relationship between IgE antileishmanial antibodies in serum and the number of leishmanial skin ulcers. It is well known that IgE develops its biologic antibody function after fixing the high-affinity receptor FCɛRI on mast cells, basophils, and Langerhans cells, and the presence of IgE antibodies in CL could be interpreted as a protective mechanism to hinder cutaneous dissemination of the infection and to modulate the skin inflammatory response mediated by Th1 cytokines. The positive correlation between IgE antileishmanial antibodies and the diameter for the skin Montenegro test could be attributed to a synergistic effect of the late-phase inflammation originating with immune reaction of leishmanial antigens injected in the skin with specific IgE antibodies fixed on mast cells and the type IV hypersensitivity caused by this test. Finally, finding elements of the Th2 immune response in early CL, demonstrated by serum IgE antileishmanial antibodies and high concentrations of this immunoglobulin in the sera of infected patients (21), is in accordance with previous observations of IL-4 and IL-10 mRNA in cells from biopsies of leishmanial skin ulcers (3, 11, 19, 23). Previous study has shown a preferential IL-10 production to the detriment of IFN-γ in early CL, with the change of this pattern occurring during the evolution of the infection, where the proinflammatory Th1 cytokine predominates (24). Apparently, the basis of the IgE stimulation and production in early CL here reported will be better understood through the elucidation of the importance of leishmanial protease antigens in the stimulation of this isotype of antibody. In atopic diseases the enzyme allergens are implicated in cleavage of CD23, the low-affinity receptor of IgE (FCɛ RII), which provokes maintenance of the Th2 immune response (25). At present, we already have verified the recognition of the purified leishmanial metallopeptidase gp63 as an antigen by serum IgE antibodies from CL patients (unpublished data), it being probably the 60-kDa protein most frequently recognized in Western immunoblotting by the CL sera evaluated in this work, and we are investigating the biological role of this immune event in CL immunopathogenesis.
This work was supported by PRONEX, National Institutes of Health grant AI-30639, and Superintendência de Apoio ao Desenvolvimento de Ciê ncia e Tecnologia (CADCT).
REFERENCES
- 1.Anam, K., F. Afrin, D. Banerjee, N. Pramanik, S. K. Guha, R. P. Goswami, P. N. Gupta, S. K. Saha, and N. Ali. 1999. Differential decline in Leishmania membrane antigen-specific immunoglobulin G (IgG), IgM, IgE, and IgG subclasses antibodies in Indian Kal-Azar patients after chemotherapy. Infect. Immun. 67:6663–6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atta, A. M., Jr., A. D’Oliveira, J. Correa, M. L. B. Atta, R. P. Almeida, and E. M. Carvalho. 1998. Anti-leishmanial IgE antibodies: a marker of active disease in visceral leishmaniasis. Am. J. Trop. Med. Hyg. 59:426–430. [DOI] [PubMed] [Google Scholar]
- 3.Cáceres-Dittmar, G., F. J. Tapia, M. A. Sánchez, M. Yamamura, K. Uyemura, R. L. Modlin, B. R. Bloom, and J. Convit. 1993. Determination of the cytokine profile in American cutaneous leishmaniasis using the polymerase chain reaction. Clin. Exp. Immunol. 91:500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho, E. M., R. S. Teixeira, and W. D. Johnson. 1981. Cell-mediated immunity in American visceral leishmaniasis: reversible immunosuppression during acute infection. Infect. Immun. 33:498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho, E. M., R. Badaró, S. G. Reed, T. C. Jones, and W. D. Johnson. 1985. Absence of gamma-IFN and interleukin-2 production during active visceral leishmaniasis. J. Clin. Investig. 76:2066–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carvalho, E. M., W. D. Johnson, E. Barreto, P. D. Marsden, J. L. M. Costa, S. Reed, and H. Rocha. 1985. Cell-mediated immunity in American cutaneous and mucosal leishmaniasis. J. Immunol. 135:4144–4148. [PubMed] [Google Scholar]
- 7.Carvalho, E. M., A. Barral, D. Pedral-Sampaio, M. Barral Netto, R. Badaró, H. Rocha, and W. D. Johnson. 1992. Immunological markers of clinical evolution in children recently infected with Leishmania donovani chagasi. J. Infect. Dis. 165:535–540. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho, E. M., A. Barral, J. M. L. Costa, A. Bittencourt, and P. Marsden. 1994. Clinical and immunopathological aspects of disseminated cutaneous leishmaniasis. Acta Trop. 56:315–325. [DOI] [PubMed] [Google Scholar]
- 9.Castes, M., A. Agnelli, O. Verde, and A. J. Rondon. 1983. Characterization of the cellular immune response in American cutaneous leishmaniasis. Clin. Immunol. Immunopathol. 27:176–186. [DOI] [PubMed] [Google Scholar]
- 10.Frey, A., J. Di Canzio, and D. Zurakowski. 1998. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods 221:35–41. [DOI] [PubMed] [Google Scholar]
- 11.Gaafar, A., B. Veress, H. Permin, A. Kharazmi, T. G. Theander, and A. M. el Hassan. 1999. Characterization of the local and systemic immune responses in patients with cutaneous leishmaniasis due to Leishmania major. Clin. Immunol. 91:314–320. [DOI] [PubMed] [Google Scholar]
- 12.Galvão-Castro, B., J. A. Sa Ferreira, K. F. Marzochi, M. C. Marzochi, S. G. Coutinho, and P. H. Lambert. 1984. Polyclonal B cell activation, circulating immune complexes and autoimmunity in human American visceral leishmaniasis. Clin. Exp. Immunol. 56:58–66. [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzel, F. P., M. D. Sadick, S. S. Mutha, and R. M. Locksley. 1991. Production of interferon-gamma, interleukin-2, interleukin-4 and interleukin-10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc. Natl. Acad. Sci. USA 88:7011–7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holaday, B. J., M. L. Pompem, S. Geromino, M. J. Teixeira, A. Q. Souza, A. W. Vasconcelos, R. D. Pearson, J. S. Abrams, and R. M. Locksley. 1993. Potential role for interleukin-10 in the immunosuppression associated with kala-azar. J. Clin. Investig. 92:2626–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter, C. A., and S. L. Reiner. 2000. Cytokines and T cells in host defense. Curr. Opin. Immunol. 12:413–418. [DOI] [PubMed] [Google Scholar]
- 16.Kane, M. M., and D. M. Moner. 2000.Leishmania parasites and their ploys to disrupt macrophage activation. Curr. Opin. Hematol. 7:26–31. [DOI] [PubMed] [Google Scholar]
- 17.Laemmly, U. K. 1970. Cleavage of the head of Bacteriophage. Nature 227:680–685. [DOI] [PubMed] [Google Scholar]
- 18.Matthews, D. J., C. L. Ericson, G. J. Mackenzie, H. E. Jolin, J. M. Blackwell, and A. N. Mackenzie. 2000. IL-13 is a susceptibility factor for Leishmania major infection. J. Immunol. 164:1458–1462. [DOI] [PubMed] [Google Scholar]
- 19.Melby, P. C., F. J. Andrade-Narvaez, B. J. Darnell, G. Valencia-Pacheco, V. V. Tryon, and A. Palomo-Cetina. 1994. Increased expression of proinflammatory cytokines in chronic lesions of human cutaneous leishmaniasis. Infect. Immun. 62:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neva, F. A. 1992. Leishmaniasis, p.1982–1987. In J. B. Wyngaarden, L. H. Smith, Jr., and J. C. Bennett (ed.), Cecil textbook of medicine, 19th ed. Saunders Company, London, United Kingdom.
- 21.O’Neil, C. E., M. Labrada, and N. G. Saraiva. 1993. Leishmania (viannia) panamensis-specific IgE and IgA antibodies in relation to expression of human tegumentary leishmaniasis. Am. J. Trop. Med. Hyg. 49:181–188. [DOI] [PubMed] [Google Scholar]
- 22.Perlmann, H., H. Helmby, M. Hagstedt, J. Carlson, P. H. Larson, M. Troye-Blomberg, and P. Perlman. 1994. IgE elevation and IgE anti-malarial antibodies in Plasmodium falciparum malaria: association of high IgE levels with cerebral malaria. Clin. Exp. Immunol. 97:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pirmez, C., M. Yamamura, K. Uyemura, M. Paes-Oliveira, F. Conceição-Silva, and R. L. Modlin. 1993. Cytokine patterns in the pathogenesis of human leishmaniasis. J. Clin. Investig. 91:1390–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocha, P. N., R. P. Almeida, O. Bacellar, A. R. de Jesus, D. C. Filho, A. C. Filho, A. Barral, R. L. Coffman, and E. M. Carvalho. 1999. Down-regulation of Th1 type of response in early human American cutaneous leishmaniasis. J. Infect. Dis. 180:1731–1734. [DOI] [PubMed] [Google Scholar]
- 25.Shakib, F., O. Schulz, and H. Sewell. 1998. A mite subversive: cleavage of CD23 and CD25 by Der p 1 enhances allergenicity. Immunol. Today 19:313–316. [DOI] [PubMed] [Google Scholar]
- 26.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei, X. Q., B. P. Leung, W. Niedbala, D. Piedrafita, G. J. Feng, M. Sweet, L. Dobbie, A. S. Smith, and F. Y. Liew. 1999. Altered immune responses and susceptibility to Leishmania major and Staphylococcus aureus infection in IL-18 deficient mice. J. Immunol. 163:2821–2828. [PubMed] [Google Scholar]
- 28.Wong, S. Y., M. Hajdu, R. Ramirez, P. Thulliez, R. McLeod, and J. S. Remington. 1993. Role of specific immunoglobulin E in diagnosis of acute Toxoplasma infection and toxoplasmosis. J. Clin. Microbiol. 31:2952–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwingenberger, K., G. Harms, C. Pedrosa, S. Omena, B. Sendkamp, and S. Neiger. 1990. Determinants of the immune response in visceral leishmaniasis: evidence for predominance of endogenous IL-4 over IFN-gamma production. Clin. Immunol. Immunopathol. 57:242–249. [DOI] [PubMed] [Google Scholar]




