Abstract
The subiculum is the first output structure distal to the hippocampus, abutting subfield CA1. As such, the subiculum receives afferent input from the hippocampus. Accumulating clinical and experimental evidence suggests that the subiculum plays an important role in the initiation and maintenance of epileptic discharges in temporal lobe epilepsy. This review discusses the anatomy and physiology of the subiculum and examines its participation in epilepsy and epileptogenesis.
Clinical, pathological, and physiological studies of focal epilepsy of temporal lobe origin have historically emphasized the role of the hippocampus. Certainly, damage in the hippocampus proper (dentate gyrus, hilus, and cornu ammonis [CA] or “Ammon's horn”) is central to temporal lobe epilepsy (TLE), with cell loss and gliosis of CA1 and CA3 subfields and the dentate hilus representing the pathologic hallmarks of the syndrome. However, emerging pathophysiological and imaging evidence strongly implicates a critical role for distal structures, including the subiculum and parahippocampal region, in TLE as well (1–4). Although cell loss in the subiculum is not a significant feature of epilepsy, the proximity of the subiculum to sites of hippocampal damage could endow it with a unique functional role in epileptogenesis.
The subiculum, located between the hippocampus proper and the parahippocampal region, represents an anatomic transition zone between Ammon's horn and the entorhinal cortex. The subiculum is the major output of the hippocampus and the first brain region encountered by neural activity emanating from the hippocampus. With this privileged location, the subiculum is exquisitely poised to modulate normal and abnormal neuronal firing as it propagates from the hippocampus to other cortical and subcortical regions.
This review summarizes the anatomy and physiology of the subiculum and correlates this information with its known and possible roles in epilepsy. The sum of evidence suggests that the subiculum is not merely “the back door” out of the hippocampus, but a dynamic and interactive structure that processes and modifies epileptic discharges and in turn is modified by them. After a summary of the anatomy and physiology of the subiculum, exciting new findings about the subiculum's role in epilepsy and epileptogenesis are discussed.
Subiculum: Anatomy and Physiology
Hippocampal Anatomy
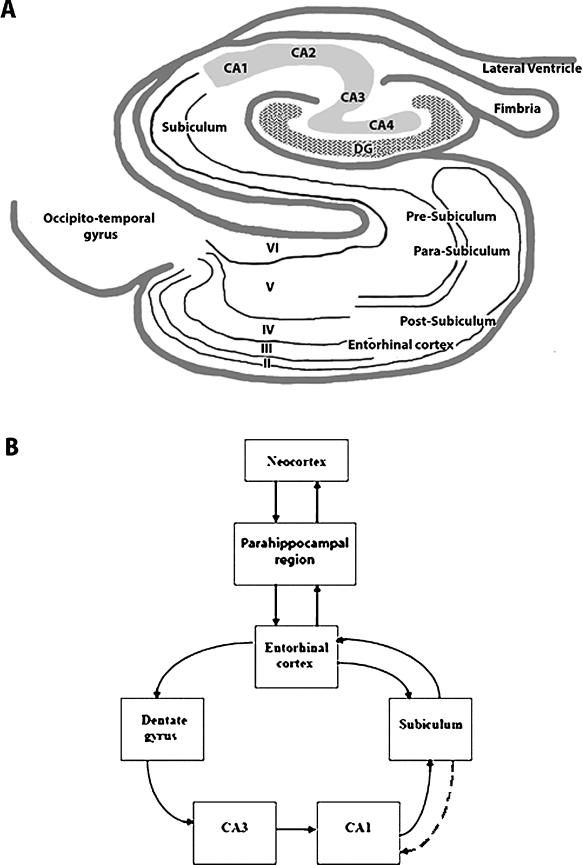
The hippocampal region consists of the hippocampal formation and parahippocampal region (Figure 1A). The hippocampal formation comprises the dentate gyrus, Ammon's horn, and the subiculum. In this review, the term hippocampus (or hippocampus proper) refers to the dentate gyrus and Ammon's horn, whereas subiculum refers to the distinct hippocampal subregion distal to the CA1 field. The word subiculum means “support” in Latin. The subiculum forms the transition zone between the hippocampus and entorhinal cortex (5). Like the hippocampus, the subiculum is three-layered allocortex (“old” cortex), consisting of a molecular layer, pyramidal cell layer, and polymorphic/fiber layer. The molecular layer is contiguous with the stratum lacunosum-moleculare and stratum radiatum of CA1. The cell layer contains large pyramidal neurons, less densely packed than in CA1, as well as variously shaped, smaller interneurons that are presumably GABAergic inhibitory cells.
FIGURE 1.
A, Schematic of a transverse section of the hippocampus and parahippocampal region. The subiculum occupies a central position between hippocampus proper and parahippocampal structures. Entorhinal cortex layers are identified with Roman numerals. DG, dentate gyrus; CA, cornu ammonis. B, Selected connections between hippocampal and parahippocampal regions. Note that hippocampal areas (three-layered cortex) tend to project unidirectionally, whereas parahippocampal areas (four or more layers) project to multiple targets. Dashed line from subiculum to CA1 indicates one possible mechanism of epilepsy-induced plasticity, whereby excitatory connections between these two areas may be strengthened (e.g., see refs. 39 and 44).
The parahippocampus or parahippocampal region is a transition zone (periallocortex) between three-layered allocortex and six-layered neocortex. The parahippocampal region consists of the presubiculum, parasubiculum, entorhinal cortex, perirhinal cortex, and, more posteriorly, the postrhinal cortex (in nonprimate mammals) or parahippocampal cortex (in primates) (6–8). At the border of the three-layered subiculum and the presubiculum, an additional cell layer appears, positioned more superficially and separated from the underlying continuation of the three-layered subicular lamination by a cell-free zone known as the lamina dessicans. As the entorhinal cortex gives way to the postrhinal/parahippocampal cortex, the lamina dessicans disappears, and a more homogeneously layered cortex (proisocortex) appears that resembles six-layered neocortex, except that it lacks an inner granular cell layer (layer IV) (9). An important feature of the connectivity of the hippocampal region is that three-layered structures project unidirectionally, whereas parahippocampal structures with more than three layers have multidirectional, reciprocal interconnections to the hippocampal formation and other parahippocampal regions (Figure 1B).
Hippocampal Circuit
The well-known trisynaptic circuit of the hippocampus consists of three defined synaptic relay stations. First, input from the entorhinal cortex enters the hippocampus via the perforant path by synapsing onto dendrites of dentate granule cells in the outer two thirds of the dentate molecular layer. Dentate granule cells have been proposed to form a “gate” or filter of activity entering the hippocampus. Next, the axons of dentate granule cells (“mossy fibers”) innervate hilar interneurons and CA3 pyramidal cells. Finally, via the Schaffer collateral pathway, CA3 neurons synapse onto CA1 pyramidal cells. The output of CA1 goes to the subiculum, and from the subiculum, activity exits the hippocampus to target entorhinal cortex and more distant subcortical and cortical areas. The use of glutamate in many of these pathways, and firing characteristics of target cells, predispose the hippocampal circuit to reverberating neuronal firing, which has been considered to be conducive to paroxysmal, epileptiform firing.
Inputs to the Subiculum
The subiculum receives dual afferent inputs from CA1 pyramidal neurons and from entorhinal cortex layer II/III neurons. The organization of CA1 afferents to the subiculum is governed by several anatomic principles (10). First, CA1-to-subiculum inputs are organized topographically. Fibers from more proximal CA1 fibers (closest to CA2) innervate the distal subiculum, whereas distal CA1 (closest to the subiculum) innervates the proximal subiculum. Second, extensive longitudinal divergence exists from CA1 to subiculum, with a given CA1 area making synaptic contacts over approximately one third of the longitudinal extent of the subiculum. Third, CA1 terminals are distributed throughout the stratum pyramidale and stratum moleculare of the subiculum, allowing CA1 to influence proximal dendrites, somata, and basal dendrites. This lamellar pattern of the CA1-to-subiculum pathway approximates a “column,” an organizational feature that becomes important when considering how the subiculum modulates epileptic discharges (see the following).
The entorhinal input to subiculum also might be important functionally for modulation of epileptic activity. Entorhinal cortex innervates the hippocampal dentate gyrus, via “ perforant path” fibers that traverse the subiculum. In addition, entorhinal cortex directly innervates the subiculum (7,11). These multiple routes of subicular innervation from entorhinal cortex, both directly and via CA1, converge onto the subiculum and might allow the amplification and modulation of incoming information.
Outputs of the Subiculum
The subiculum innervates a wide array of targets. Fibers from the subiculum travel via the fornix to medial and lateral mammillary nuclei, ventral hypothalamus, midline thalamic nuclei, the lateral septal nucleus, and the nucleus accumbens (12). Other subiculum outputs reach the retrosplenial cortex, entorhinal cortex, peri- and postrhinal cortices, presubiculum, and parasubiculum via nonfornix projections (8,13). Subiculum-to-entorhinal cortex pathways also are organized topographically. The proximal subiculum and adjacent CA1 innervate the lateral entorhinal cortex, whereas the distal subiculum and proximal CA1 innervate the medial entorhinal cortex. The existence of a reciprocal excitatory CA1–subiculum pathway raises the possibility of an anatomic substrate for hyperexcitability (14).
In summary, the input and output pathways of the subiculum are hodologically complex (8,12). (Hodologic is a little-known word referring to “pathways.”) Hippocampus–subiculum–entorhinal cortex pathways entail several parallel closed or “nested” loops, with the input topography related to the precise site of origin of the innervation. The importance of this complicated three-dimensional matrix likely has important functional consequences in both normal and abnormal neuronal firing.
Cell Types and Physiology of Neurons in the Subiculum
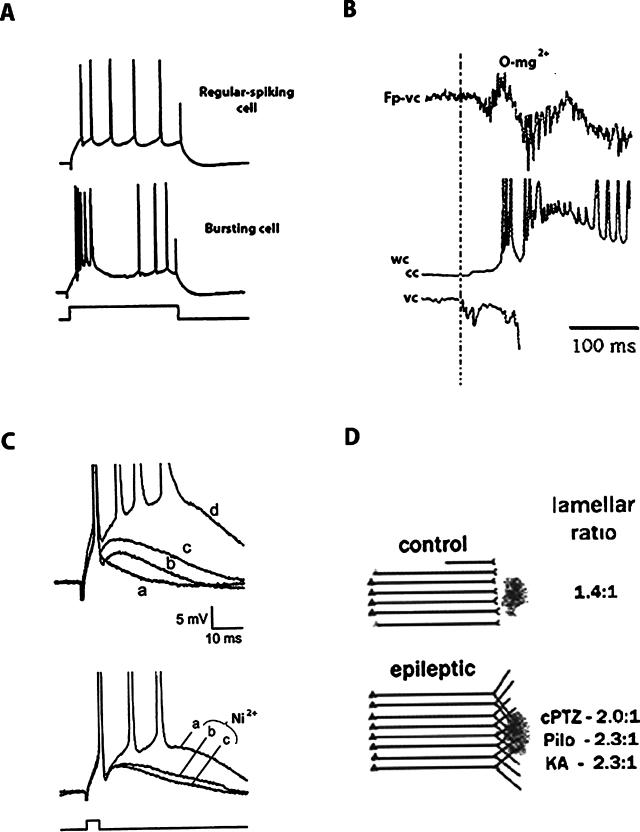
The subiculum, like the hippocampus proper, consists of three-layered archicortex. Neurons of the subiculum include pyramidal (principal) cells and a variety of smaller interneurons. Subicular pyramidal cells have been classified into bursting neurons and regular-spiking neurons (Figure 2A) (15–19). Bursters fire multiple action potentials (usually three to five) in response to orthodromic stimulation or the initial portion of a depolarizing current pulse, whereas regular-spiking neurons fire a regular train of action potentials for the duration of the stimulus. The ionic mechanisms underlying bursting neuronal behavior are not fully clarified, but bursting is clearly dependent on sodium and calcium conductances (16,20). Many of the intrinsic membrane and cable properties of bursters and regular-firing cells are similar, but bursters have slightly more negative resting potentials; when depolarized, bursters can be induced to fire in the regular-spiking mode (16).
FIGURE 2.
A, Intracellular recordings from two subicular neurons, a regular-spiking neuron (top) and a bursting neuron (middle), in response to a depolarizing current pulse (bottom). [Reproduced with permission from ref. 19, p. 260]. B, Field (Fp) and patch-clamp recordings from a subiculum slice bathed in zero magnesium, showing the appearance of synchronized epileptiform field activity (top), and simultaneous whole-cell recordings in current-clamp (cc, middle) and voltage-clamp (vc, bottom) modes. The illustrated traces indicated “widespread activity” (see text). Vertical dashed line, The beginning of bursting neuron activity, which fires before the development of epileptiform field activity about 25 msec later. These findings indicate that, during widespread epileptiform activity, the burster cell is the leader cell. Later (not shown here), neural firing begins in follower cells such as fast-spiking interneurons and regular spiking neurons. These results led the authors to propose a sequence of neuron recruitment in epileptic subiculum, with burster cells playing a prominent initiating role (see text). Reproduced in modified form with permission from ref. 29, p. 5529. C, Intracellular recordings from bursting neurons in slices from subiculum. Traces illustrate differences in the afterdepolarizations between a control nonbursting neuron (a), a control high-threshold bursting neuron (b), and two low-threshold bursters from rats that had undergone pilocarpine-induced status epilepticus several weeks earlier (c, d). Bursting cells with lower burst-firing thresholds and in epileptic rats had the largest ADPs. Reprinted with permission from ref. 19, p. 262. Bottom traces, Effect of extracellular nickel application to block calcium current in a bursting neuron from epileptic subiculum. The largest ADP is seen in the subicular bursting neuron in control artificial cerebrospinal fluid (a). The ADP size is reduced at two time points (10 minutes, b, and 14 minutes, c) after extracellular application of Ni2+. Therefore, bursting capacity is dependent on calcium influx. Reproduced with permission from ref. 19, p. 263. D, Diagram showing epilepsy-induced plasticity (sprouting) of CA1 terminals into subiculum. In the control situation, CA1 axons innervate subiculum in a lamellar fashion. The dark blob indicates the site of sodium selenite injection into the subiculum. This retrograde marker labels CA1 terminals. In several models of chronic epilepsy, synaptic rearrangement (sprouting) of CA1 terminals is indicated by marked expansion of selenite uptake, diagrammatically indicated here by the expanded CA1 terminal distribution. The lamellar ratios indicate the ratio of dorsoventral extent of the retrogradely neurons to the injection site. Lamellar ratios are significantly increased in the three epilepsy models shown, suggesting expansion of the CA1 terminal fields (sprouting). cPTZ, chronic pentylenetetrazole; Pilo, pilocarpine; KA, kainic acid. [Reproduced with permission from ref. 44, p. 685].
In normal subiculum, the proportion of bursting neurons and regular-spiking neurons varies, but it is generally agreed that bursting neurons outnumber regular-spiking cells, by some estimates as much as 2:1. Bursting neurons may be more prominent in deeper subicular layers (18) and farther away from the CA1 border (17). The presence of bursting cells increases the likelihood of synaptic release and dendritic back-propagation that may alter integrative properties of the neuron (17). Bursting neurons have been subclassified into weak and strong bursters, which differ in terms of their firing thresholds (17,21).
By comparison, regular-spiking subicular neurons, like neighboring CA1 pyramidal neurons, ordinarily fire in single spikes, even in response to prolonged depolarizing current pulses. Regular-spiking subicular cells and CA1 pyramidal neurons can convert into a burst-firing mode under certain conditions such as the application of the D-type potassium channel blocker 4-aminopyridine (17).
Bursting and regular-spiking subicular neurons are glutamatergic projection cells. The spatiotemporal aspects of firing are controlled by GABAergic inhibitory neurons, many of which are fast-spiking interneurons. As is discussed in subsequent sections, it is intriguing to speculate that the physiology or distribution of subicular neurons and their subtypes could determine the pattern of epileptiform firing in the subiculum.
Functions of the Subiculum
The subiculum is an integral part of the limbic memory system, responsible for memory retrieval (22) and spatial encoding (23–26). Similar to CA1 neurons, subicular neurons are place cells that respond when the animal is in a particular location in space. The hippocampus and subiculum act together to establish and retrieve short-term memories (27). Each structure acts within a different time frame to allow this function—the subiculum is most active at time intervals less than 10 to 15 seconds, whereas the hippocampus acts at times longer than 15 seconds (28). Therefore, it is thought that the subiculum is most important for retrieval of newly learned information, but its detailed role in memory and cognition, and whether its function is altered in epilepsy, remain to be determined.
Subiculum Circuitry and Physiology Predispose to Hyperexcitability
Although several physiological classes of subicular neurons have been identified (bursting, regular-spiking, and fast-spiking neurons), it is not known how these neurons integrate into a functional network and how they generate epileptiform firing. The presence of bursting neurons certainly suggests that the subiculum can fire in an epileptic mode. Bursting in subicular neurons is dependent on both intrinsic and synaptic conductances. Bursting is increased by picrotoxin (a GABA blocker) and decreased by N-methyl-d-aspartate–receptor blockers.
To investigate the ability of subicular neurons to produce epileptic activity, field recordings were made from subicular slices bathed in zero-magnesium medium to enhance N-methyl-d-aspartate–mediated excitation (29). The contribution of different cell types to epileptic field activity was studied by using concurrent patch and field recordings. Three distinct types of bursting fields were observed: 1) desynchronized firing, 2) focal firing, and 3) widespread firing. During desynchronized firing, irregular cell firing was seen, mostly in fast-spiking interneurons and intrinsic bursters. By using a quantitative measure of firing, a “driving index” was derived, which estimated the probability that a cell would start firing before the field. In focal field activity, bursters began to fire before the paroxysmal field activity, whereas regular-spiking neurons and fast-spiking neurons fired after field activity began; firing of fast-spiking interneurons probably prevented spread of the activity beyond the focus. Therefore, bursting subicular neurons may initiate the focal epileptiform activity. Focal activity is dependent on N-methyl-d-aspartate activity, and spatial specificity is strongly determined by local inhibition.
When widespread epileptiform activity was recorded, similar results were found: bursters fired 50 to 100 msec before the epileptic field activity commenced, and burster firing peaked just as the field activity began (Figure 2B). Regular-spiking neurons fired after the field activity began, and the timing of firing of fast-spiking interneurons was variable, either before or after the field activity.
The authors concluded that firing capacity in the subiculum is determined by both intrinsic properties and morphology. Driving cells had more inputs on distal dendrites than did follower cells. A sequence of neuron recruitment in the subiculum was postulated that could lead to epileptiform activity (29). Bursters have the lowest firing threshold and were often the “leader cells”; they possess a greater proportion of inputs on distal dendrites than do follower cells. Follower cells are either excitatory or inhibitory. Inhibitory follower cells, which use fast GABA inhibition, feed back onto the leader cells to attenuate their activity. Fast inhibition can prevent the focal activity from becoming widespread, thereby sculpting the activity of the system.
Interictal Epileptiform Discharges in Patients with Temporal Lobe Epilepsy Originate in the Subiculum
Interestingly, numerous investigations of hippocampal tissue resected from patients with TLE have revealed little spontaneous firing that could be considered a pathophysiologic hallmark of epilepsy. In such tissue, hyperexcitability is observed mainly when the system is manipulated pharmacologically (e.g., by blocking GABA receptors). A possible reason for the lack of observable spontaneous epileptiform activity is that excessive hippocampal cell death prevents synchronization of neuronal firing in hippocampus proper, yet seizures of hippocampal origin do occur in TLE, so the question arises as to exactly where interictal and ictal discharges originate. Relatively little cell loss occurs in the subiculum of patients with TLE (30,31). This observation, in conjunction with the close physical proximity of the subiculum to CA1 and the presence of intrinsic bursting cells in the subiculum, raises the possibility that the subiculum might participate in abnormal epileptic firing in TLE. To evaluate the hypothesis that subicular neurons could serve as an origin of epileptiform activity, tissue from 21 patients with mesial temporal sclerosis and refractory temporal lobe seizures was studied in vitro (32). Multielectrode recordings identified spontaneous rhythmic spikes and epileptiform field potentials in subiculum but not in the hippocampus proper. The epileptic activity originated in the subiculum and then propagated to the hippocampus. (Propagation of epileptiform activity from subiculum to CA1 also has been shown in hippocampal slices from the rat [33].) Subicular interneurons fired before and during interictal spikes on EEGs, a mechanism that was proposed to synchronize the activity of pyramidal neurons. The population of subicular neurons that gave rise to this abnormal activity was not fixed but varied over time, suggesting that no specific group of “epileptic neurons” exists; rather, different cells can take on this role at different times.
A distinct subpopulation of subicular pyramidal neurons (22% of the total) responded to GABAergic interneuron input with depolarizing responses, rather than with expected hyperpolarizing responses. Cohen et al. (32) speculated that these depolarizing GABAergic responses endowed those pyramidal neurons with properties that favored epileptic firing. The explanation for the depolarizing GABA responses was presumed to be similar to the developmentally regulated GABA excitatory phenomenon seen early in ontogeny, that is, an abnormal chloride gradient causing the chloride reversal potential to reside at a depolarized level relative to resting potential (34). It is unknown how widespread this phenomenon is, whether it is mediated by specific GABA-receptor subtypes, and whether it is due to neuronal injury. Clearly, however, our understanding of GABA mechanisms in normal and abnormal neuronal function is expanding beyond a simple inhibitory role (35).
Another study using human resected TLE tissue found altered subicular neuron firing that correlated with EEG spikes, but depolarizing GABA responses were not observed (36). As in the Cohen report (32), the majority of the recorded cells were regular-spiking neurons. No major physiologic differences were found in tissue exhibiting severe mesial temporal sclerosis and less severe sclerosis. It was concluded that hippocampal sclerosis and consequent subicular deafferentation are not required for development of an epileptic focus in the subiculum. Technical differences between the two studies could account for the disparities in terms of GABAergic signaling (37). In any case, it is clear that the subiculum exhibits hyperexcitability and excessive synchrony in human TLE.
Epilepsy-induced Alteration of Subicular Neuron Firing Properties
To investigate the epilepsy-induced changes in neuronal properties and firing patterns in subicular neurons, two studies used pilocarpine to induce status epilepticus in rats; cell properties were studied in vitro several weeks later. Wellmer et al. (19) found a marked increase in the percentage of burst-firing subicular neurons after status epilepticus, from about 40% of total cells in controls to 82% of cells after pilocarpine. Subicular neurons from rats that experienced status epilepticus had enhanced spike afterdepolarizations, consistent with an increase in excitability (Figure 2C, top). Postburst afterdepolarizations were dependent on the entry of calcium ions, similar to those seen in CA1 neurons after pilocarpine (38); when extracellular calcium was decreased or calcium channels were blocked with nickel, the afterdepolarizations decreased in a time- and concentration-dependent fashion (Figure 2C, bottom). The authors concluded that the increased percentage of intrinsically bursting neurons and their altered physiology accounted for the enhanced epileptogenicity of the subiculum in this model.
In the second study, the cellular and network properties of morphologically and physiologically identified subicular neurons were studied after pilocarpine-induced status epilepticus in rats that later developed spontaneous seizures (39). In control animals, regular spiking and bursting neurons were characterized by extensive axonal branching and autapse-like contacts, suggesting a high degree of intrinsic connectivity. Even in controls, some subicular neurons projected back to CA1. In epileptic tissue, spontaneous rhythmic activity, a large increase in polyphasic responses, and evoked all-or-none action-potential bursts were seen, implying enhanced network excitability. In epileptic animals, a loss was found of about 30% of subicular cells and a reversal of the ratio of bursting neurons to regular-spiking neurons from 2:1 to about 1:2. These results differ from those of Wellmer et al. (19), who found an upregulation of bursters in pilocarpine-treated animals. Both sets of authors attribute this discrepancy to the respective recording sites within the subiculum (fewer bursting neurons are expected in the proximal portion of the subiculum near the CA1 border, where the Wellmer group focused its recordings) (39).
Knopp and colleagues (36) did not observe axonal sprouting of subicular neurons and concluded that sprouting in this region was not required for the development of epilepsy. These results parallel human studies by the same group (discussed earlier), where CA1 neuron loss was not mandatory for the development of hyperexcitability in subicular neurons. They did find reduced dendritic arborization and spine density of the proximal apical dendrites of subicular neurons, suggesting a partial deafferentation from CA1.
Epilepsy-induced Synaptic Reorganization in the Subiculum
It is well established that synaptic reorganization, in the form of mossy fiber sprouting in the dentate granule molecular layer and CA3 supragranular layer, contributes to hyperexcitability in the epileptic hippocampus (40). Numerous studies, in both humans and experimental animals, have established that sprouting contributes to limbic hyperexcitability. Demonstration of sprouting in these regions is possible in part owing to the fortuitous presence of zinc in the presynaptic terminals of mossy fibers. As zinc stains black with the Timm histochemical method, it has been demonstrated that in epilepsy, mossy fiber terminals containing zinc redistribute into the outer molecular layer of the dentate gyrus, where they are normally not found. It is suspected, although hard to prove, that reorganization of synapses elsewhere in the hippocampus and beyond might also be present in the epileptic brain. In particular, sprouting of CA1 neurons has been suggested by both anatomic and physiological experiments (41–43). CA1 neurons exhibit hyperexcitability in experimental models, and it is hypothesized that synaptic reorganization underlies that increased excitability. Because the subiculum is the main outflow path of the hippocampus, sprouting of the CA1–subiculum pathway would be an attractive explanation for hyperexcitability in TLE.
This hypothesis was investigated in detailed morphologic studies in rats by using five different epilepsy models (44). Three models were short term (intraventricular kainic acid, systemic kainic acid, systemic pilocarpine) and two models were long term (kindling, prolonged intraperitoneal pentylenetetrazole). Timm staining was evaluated in the CA1 region. The source of the Timm granules was investigated by using sodium selenite injection into the subiculum, which retrogradely labels zinc-containing terminals.
In normal rats, CA1 neurons project to the subiculum topographically, in a lamellar fashion. In epileptic rats, retrograde labeling extended in a much wider distribution, crossing usual lamellar boundaries (Figure 2D). The results suggest that, in these epilepsy models, CA1 pyramidal axons sprout into the subiculum (as well as into the stratum lacunosum-moleculare of CA1). This sprouting could lead to amplification and synchronization of epileptic discharges as they emerge from the hippocampus.
Summary
The subiculum is a critical brain region in TLE. Its position as the output gate of the hippocampus allows it to modulate epileptic discharges as they exit the hippocampus proper. The presence of lamellar, topographically organized inputs from CA1, neurons with intrinsic properties that promote burst firing, and circuitry that supports synaptic reorganization in epilepsy—all confer on the subiculum a dynamic role in the modulation of epilepsy and epileptogenesis. Despite differences among studies in terms of subiculum/CA1 sprouting, the number and proportion of bursting neurons, and specific GABA-mediated functions, the subiculum possesses the potential to sustain abnormal synchrony and hyperexcitability, making it a critical region for future studies. Subsequent research may identify aspects of subicular firing that can be altered in epilepsy, paving the way for novel therapeutic interventions.
Acknowledgments
I thank Dr. Helen Scharfman and Dr. Jose Cavazos for helpful comments on the manuscript.
References
- 1.Scharfman HE, Witter MP, Schwarcz R, editors. The parahippocampal region: Implications for neurological and psychiatric diseases. Vol. 911. New York: New York Academy of Sciences; 2000. [PubMed] [Google Scholar]
- 2.Avoli M, D'Antuono M, Louvel J, Kohling R, Biagini G, Pumain R, D'Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- 3.Bernasconi N, Bernasconi A, Caramanos Z, Antel SB, Andermann F, Arnold DL. Mesial temporal damage in temporal lobe epilepsy: A volumetric MRI study of the hippocampus, amygdala, and parahippocampal region. Brain. 2003;126:462–469. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- 4.Stafstrom CE. Epileptogenesis beyond the hippocampus. Epilepsy Curr. 2003;3:66–67. doi: 10.1046/j.1535-7597.2003.03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villani F, Garbelli R, Cipelletti B, Spreafico R. The limbic system: Anatomical structures and embryological development. In: Avanzini G, Beaumanoir A, Mira L, editors. Limbic Seizures in Children. London: John Libbey; 2001. pp. 11–20. [Google Scholar]
- 6.Burwell RD, Witter MP, Amaral DG. Perirhinal and postrhinal cortices of the rat: A review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus. 1995;5:390–408. doi: 10.1002/hipo.450050503. [DOI] [PubMed] [Google Scholar]
- 7.Witter MP, Wouterlood FG, Naber PA, van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- 8.O'Mara SM, Commins S, Anderson M, Gigg J. The subiculum: A review of form, physiology, and function. Prog Neurobiol. 2001;64:129–155. doi: 10.1016/s0301-0082(00)00054-x. [DOI] [PubMed] [Google Scholar]
- 9.Scharfman HE, Witter MP, Schwarcz R. Preface. Ann N Y Acad Sci. 2000;911:ix–xiii. [PubMed] [Google Scholar]
- 10.Amaral DG, Dolorous C, Alvarez-Roy P. Organization of CA1 projections to the subiculum: A PHA-L analysis in the rat. Hippocampus. 1991;1:415–436. doi: 10.1002/hipo.450010410. [DOI] [PubMed] [Google Scholar]
- 11.Tamamaki N, Nojyo Y. Preservation of topography in the connections between the subiculum, field CA1, and the entorhinal cortex in rats. J Comp Neurol. 1995;353:379–390. doi: 10.1002/cne.903530306. [DOI] [PubMed] [Google Scholar]
- 12.Witter MP, Groenewegen HJ. The subiculum: Cytoarchitectonically a simple structure, but hodologically complex. Prog Brain Res. 1990;83:47–58. doi: 10.1016/s0079-6123(08)61240-6. [DOI] [PubMed] [Google Scholar]
- 13.Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- 14.Shao LR, Dudek FE. Electrophysiological evidence using focal flash photolysis of caged glutamate that CA1 pyramidal cells receive excitatory synaptic input from the subiculum. J Neurophysiol. December 15, 2004 doi: 10.1152/jn.00877.2004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Taube JS. Electrophysiological properties of neurons in the rat subiculum in vivo. Exp Brain Res. 1993;96:304–318. doi: 10.1007/BF00227110. [DOI] [PubMed] [Google Scholar]
- 16.Mattia D, Kawasaki H, Avoli M. In vitro electrophysiology of rat subicular bursting neurons. Hippocampus. 1997;7:48–57. doi: 10.1002/(SICI)1098-1063(1997)7:1<48::AID-HIPO5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Staff N, Jung HY, Thiagarajan T, Yao M, Spruston N. Resting and active properties of pyramidal neurons in subiculum and CA1 of rat hippocampus. J Neurophysiol. 2000;84:2398–2408. doi: 10.1152/jn.2000.84.5.2398. [DOI] [PubMed] [Google Scholar]
- 18.Harris E, Stewart M. Intrinsic connectivity of the rat subiculum, II: Properties of synchronous spontaneous activity and a demonstration of multiple generator regions. J Comp Neurol. 2001;436:506–518. doi: 10.1002/cne.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wellmer J, Su H, Beck H, Yaari Y. Long-lasting modification of intrinsic discharge properties in subicular neurons following status epilepticus. Eur J Neurosci. 2002;16:259–266. doi: 10.1046/j.1460-9568.2002.02086.x. [DOI] [PubMed] [Google Scholar]
- 20.Jung HY, Staff N, Spruston N. Action potential bursting in subicular neurons is driven by a calcium tail current. J Neurosci. 2001;21:3312–3321. doi: 10.1523/JNEUROSCI.21-10-03312.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menendez de la Prida L, Suarez F, Pozo MA. Electrophysiological and morphological diversity of neurons from rat subicular complex in vitro. Hippocampus. 2003;13:728–744. doi: 10.1002/hipo.10123. [DOI] [PubMed] [Google Scholar]
- 22.Gabrieli JDE, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- 23.Sharp E, Green C. Spatial correlates of firing patterns of single cells in the subiculum of the freely moving rat. J Neurosci. 1994;14:2339–2356. doi: 10.1523/JNEUROSCI.14-04-02339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taube JS. Place cells recorded in the parasubiculum of freely moving rats. Hippocampus. 1995;5:569–583. doi: 10.1002/hipo.450050608. [DOI] [PubMed] [Google Scholar]
- 25.Hampson RE, Hedberg T, Deadwyler SA. Differential information processing by hippocampal and subicular neurons. Ann N Y Acad Sci. 2000;911:151–165. doi: 10.1111/j.1749-6632.2000.tb06724.x. [DOI] [PubMed] [Google Scholar]
- 26.Naber A, Witter MP, Lopes da Silva FF. Networks of the hippocampal memory system of the rat. Ann N Y Acad Sci. 2000;911:392–403. doi: 10.1111/j.1749-6632.2000.tb06739.x. [DOI] [PubMed] [Google Scholar]
- 27.Miyashita Y. Cognitive memory: cellular and network machineries and their top-down control. Science. 2004;306:435–440. doi: 10.1126/science.1101864. [DOI] [PubMed] [Google Scholar]
- 28.Deadwyler SA, Hampson RE. Differential but complementary mnemonic functions of the hippocampus and subiculum. Neuron. 2004;42:465–476. doi: 10.1016/s0896-6273(04)00195-3. [DOI] [PubMed] [Google Scholar]
- 29.Menendez de la Prida L, Gal B. Synaptic contributions to focal and widespread spatiotemporal dynamics in the isolated rat subiculum in vitro. J Neurosci. 2004;24:5525–5536. doi: 10.1523/JNEUROSCI.0309-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher D, Sperber EF, Moshe SL. Hippocampal sclerosis revisited. Brain Dev. 1998;20:563–573. doi: 10.1016/s0387-7604(98)00069-2. [DOI] [PubMed] [Google Scholar]
- 31.Dawodu S, Thom M. Quantitative neuropathology of the entorhinal cortex region in patients with hippocampal sclerosis and temporal lobe epilepsy. Epilepsia. 2005;46:23–30. doi: 10.1111/j.0013-9580.2005.21804.x. [DOI] [PubMed] [Google Scholar]
- 32.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 33.Harris E, Stewart M. Propagation of synchronous epileptiform events from subiculum backward into area CA1 of rat brain slices. Brain Res. 2001;895:41–49. doi: 10.1016/s0006-8993(01)02023-6. [DOI] [PubMed] [Google Scholar]
- 34.Cohen I, Navarro V, Le Duigou C, Miles R. Mesial temporal sclerosis: A pathological replay of developmental mechanisms? Biol Cell. 2003;95:329–333. doi: 10.1016/s0248-4900(03)00081-9. [DOI] [PubMed] [Google Scholar]
- 35.Cossart R, Bernard C, Ben-Ari Y. Multiple facets of GABAergic neurons and synapses: Multiple fates of GABA signalling in epilepsies. Trends Neurosci. 2005;28:108–115. doi: 10.1016/j.tins.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Wozny C, Kivi A, Lehmann TN, Dehnicke C, Heinemann U, Behr J. Comment on “On the origin of interictal activity in human temporal lobe epilepsy in vitro.”. Science. 2003;301:463c. doi: 10.1126/science.1084237. [DOI] [PubMed] [Google Scholar]
- 37.Cohen I, Navarro V, Huberfeld G, Clemenceau S, Baulac M, Miles R. Response to comment on “On the origin of interictal activity in human temporal lobe epilepsy in vitro.”. Science. 2003;301:463d. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 38.Sanabria ERG, Su H, Yaari Y. Initiation of network bursts by Ca2+-dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. J Physiol (Lond) 2001;532:205–216. doi: 10.1111/j.1469-7793.2001.0205g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knopp A, Kivi A, Wozny C, Heinemann U, Behr J. Cellular and network properties of the subiculum in the pilocarpine model of temporal lobe epilepsy. J Comp Neurol. 2005;483:476–488. doi: 10.1002/cne.20460. [DOI] [PubMed] [Google Scholar]
- 40.Pitkanen A, Sutula T. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal lobe epilepsy. Lancet Neurol. 2002;1:173–181. doi: 10.1016/s1474-4422(02)00073-x. [DOI] [PubMed] [Google Scholar]
- 41.Esclapez M, Hirsch JC, Ben-Ari Y, Bernard C. Newly formed excitatory pathways provide a substrate for hyperexcitability in experimental temporal lobe epilepsy. J Comp Neurol. 1999;408:449–460. doi: 10.1002/(sici)1096-9861(19990614)408:4<449::aid-cne1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 42.Lehmann TN, Gabriel S, Eilers A, Njunting M, Kovacs R, Schulze K, Lanksch WR, Heinemann U. Fluorescent tracer in pilocarpine-treated rats shows widespread aberrant hippocampal neuronal connectivity. Eur J Neurosci. 2001;14:83–95. doi: 10.1046/j.0953-816x.2001.01632.x. [DOI] [PubMed] [Google Scholar]
- 43.Smith BN, Dudek FE. Network interactions mediated by new excitatory connections between CA1 pyramidal cells in rats with kainate-induced epilepsy. J Neurophysiol. 2002;87:1655–1658. doi: 10.1152/jn.00581.2001. [DOI] [PubMed] [Google Scholar]
- 44.Cavazos JE, Jones SM, Cross DJ. Sprouting and synaptic reorganization in the subiculum and CA1 region of the hippocampus in acute and chronic models of partial-onset epilepsy. Neuroscience. 2004;126:677–688. doi: 10.1016/j.neuroscience.2004.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]