Abstract
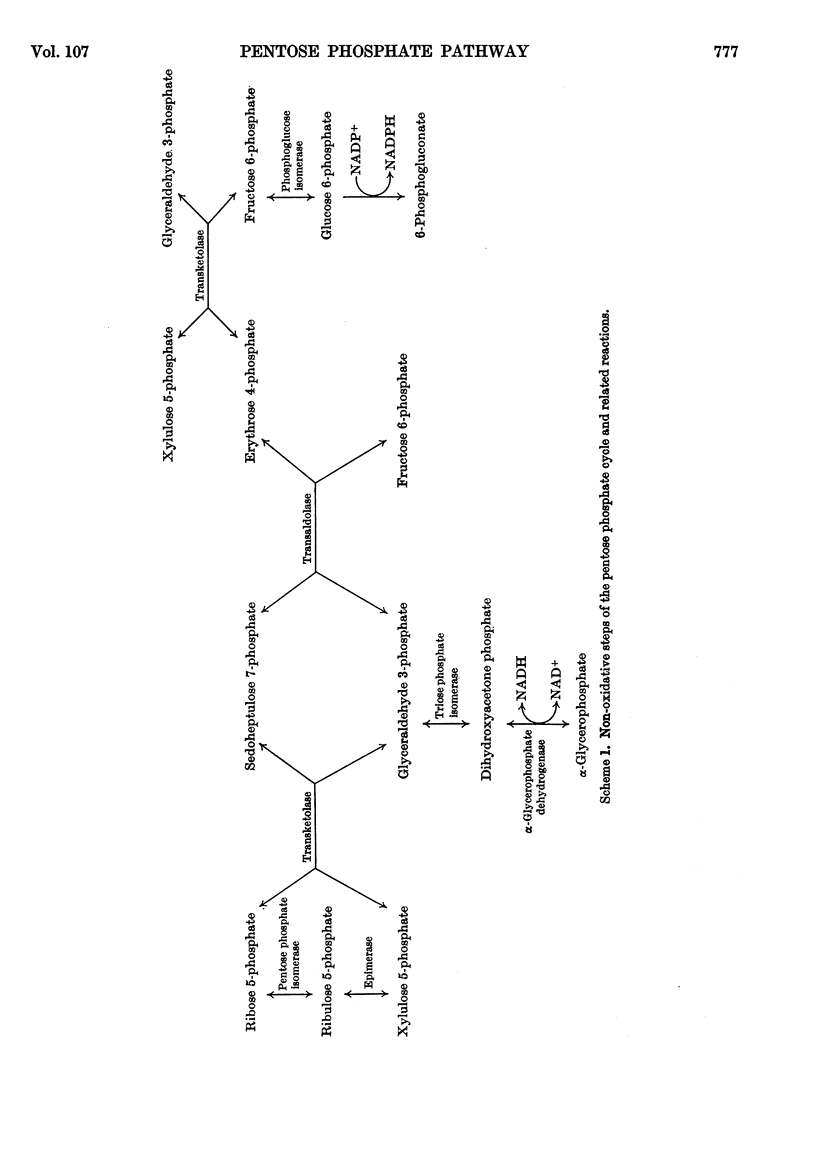
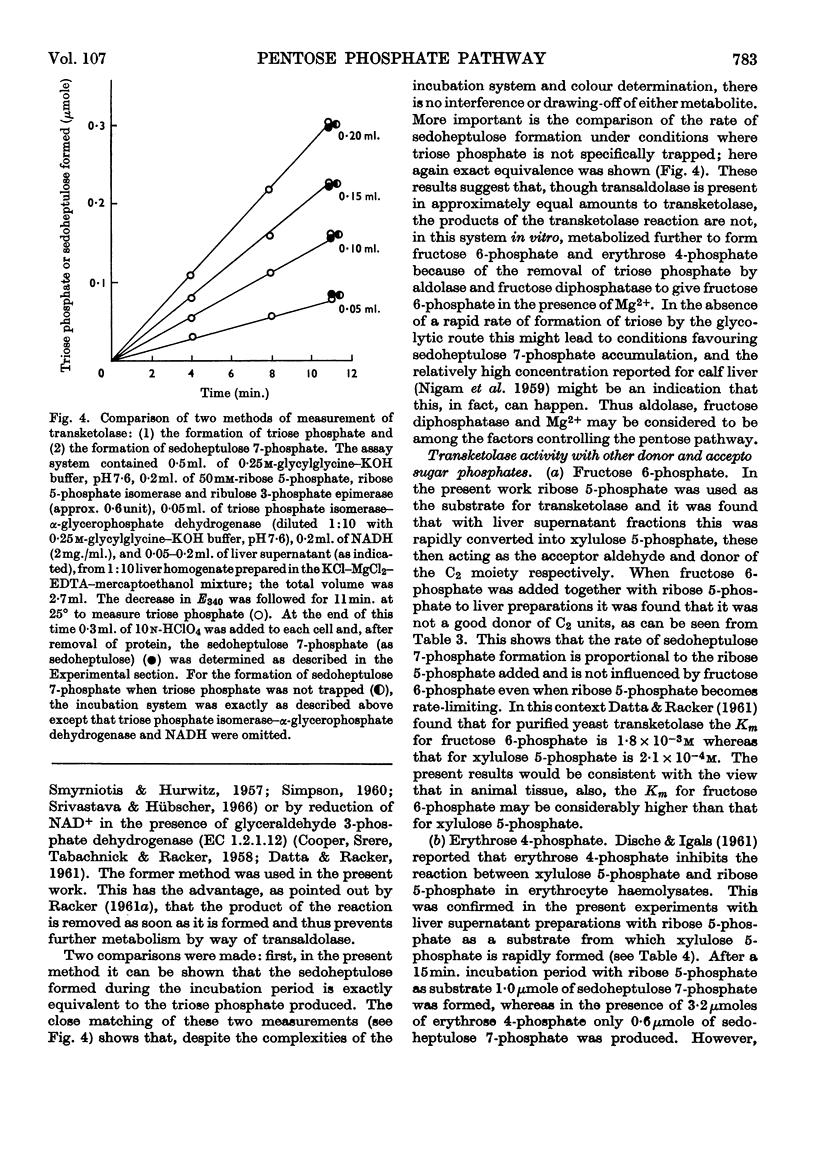
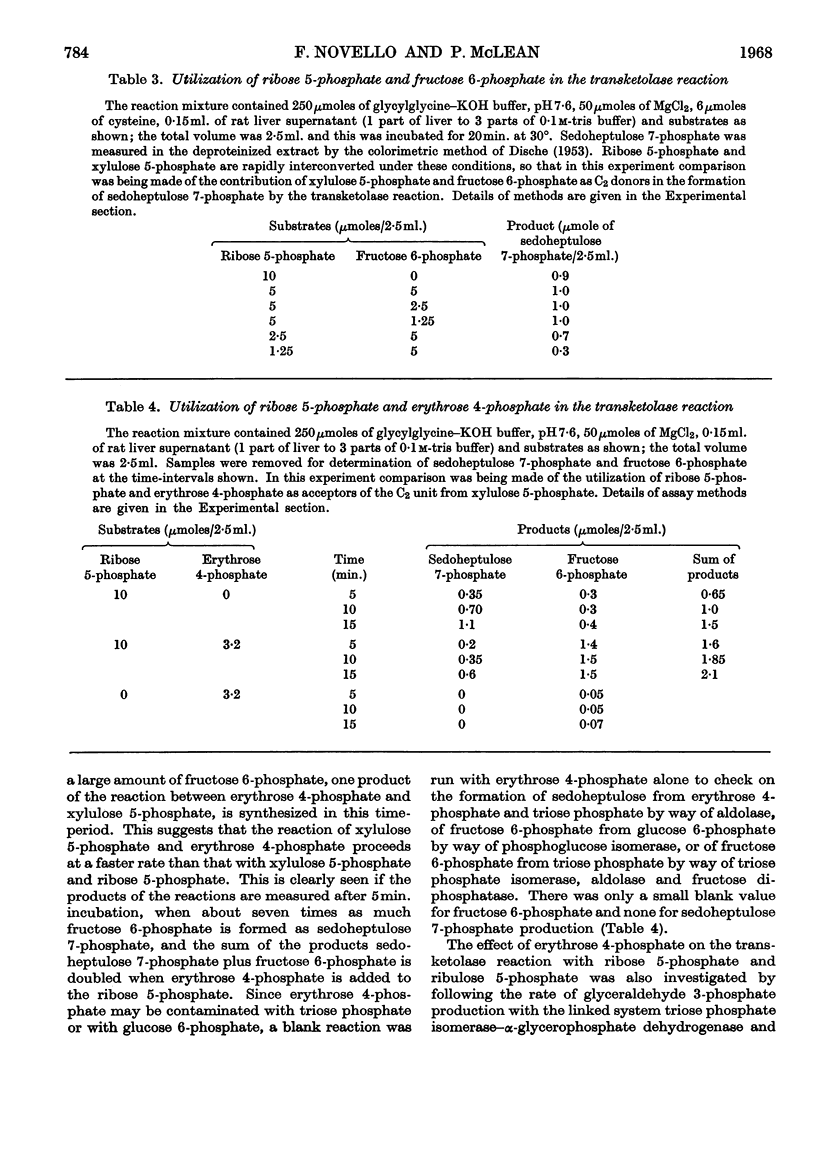
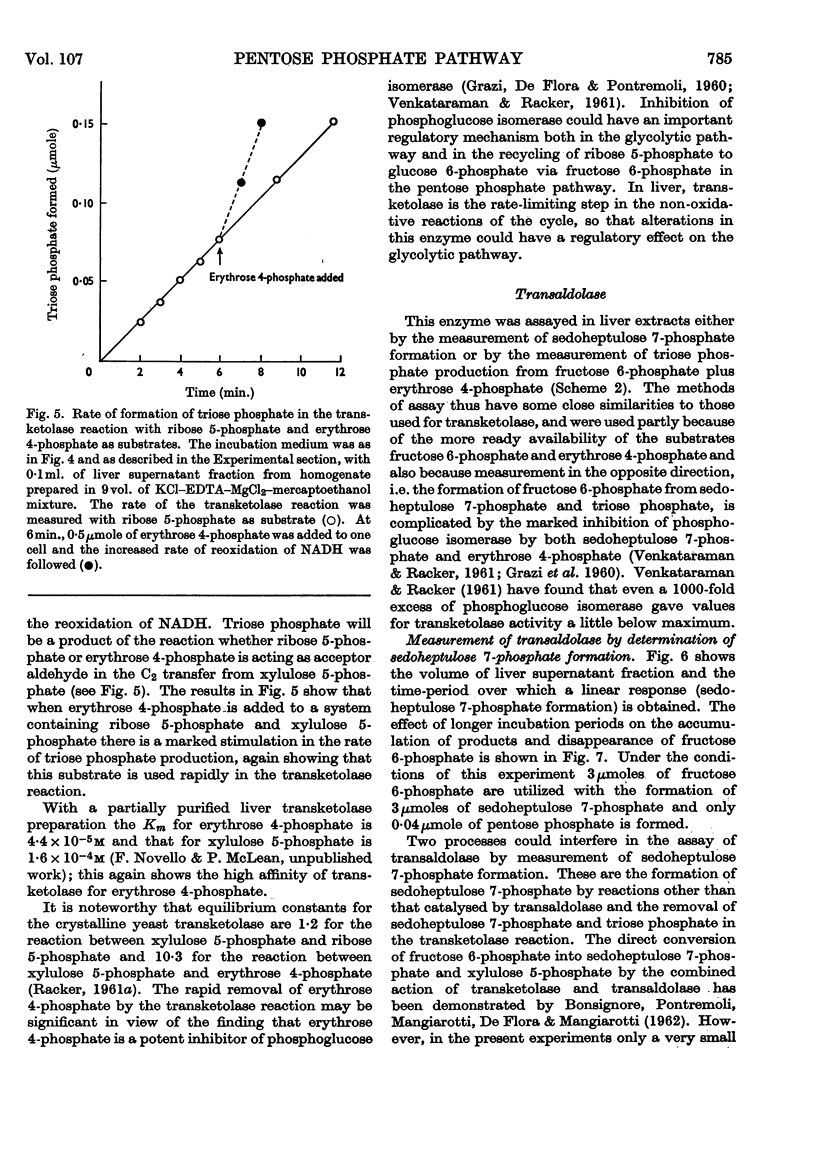
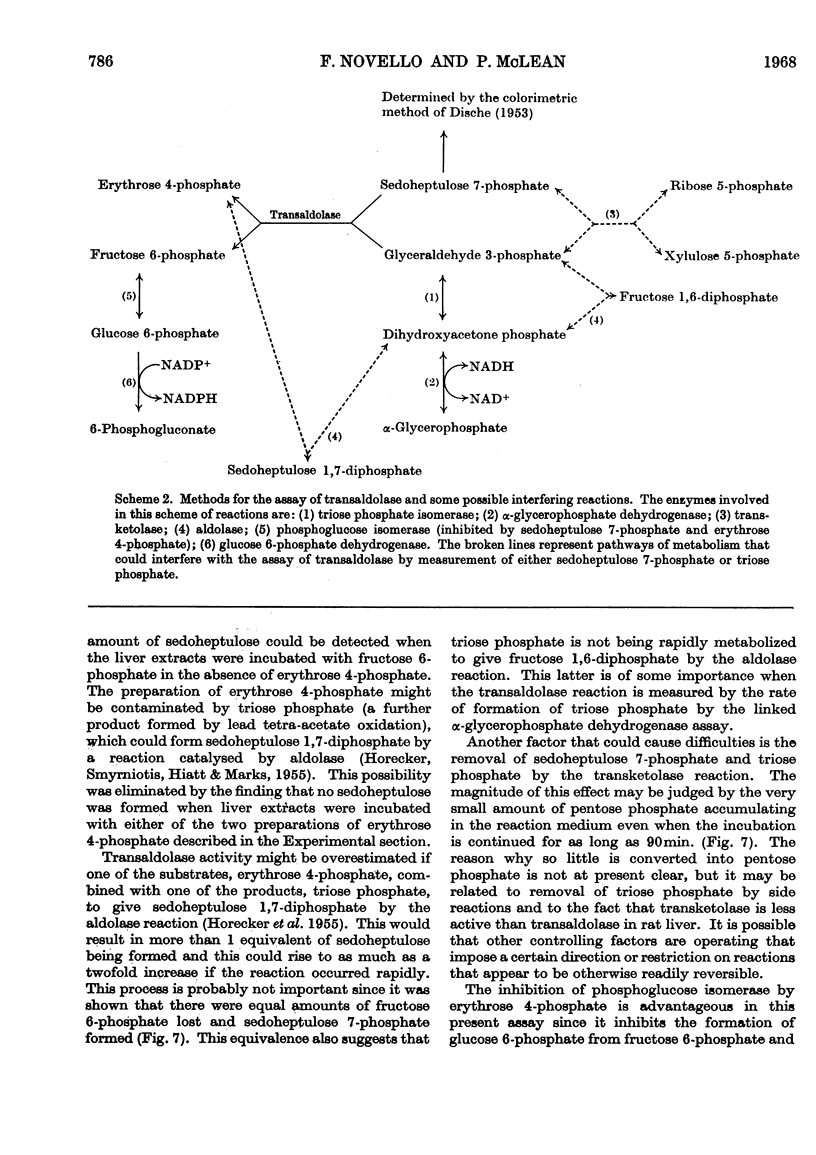
Methods for the quantitative determination of ribose 5-phosphate isomerase, ribulose 5-phosphate 3-epimerase, transketolase and transaldolase in tissue extracts are described. The determinations depend on the measurement of glyceraldehyde 3-phosphate by using the coupled system triose phosphate isomerase, α-glycero-phosphate dehydrogenase and NADH. By using additional purified enzymes transketolase, ribose 5-phosphate isomerase and ribulose 5-phosphate epimerase conditions could be arranged so that each enzyme in turn was made rate-limiting in the overall system. Transaldolase was measured with fructose 6-phosphate and erythrose 4-phosphate as substrates, and again glyceraldehyde 3-phosphate was measured by using the same coupled system. Measurements of the activities of the non-oxidative reactions of the pentose phosphate pathway were made in a variety of tissues and the values compared with those of the two oxidative steps catalysed by glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARESE P. EINE ENZYMATISCHE METHODE ZUR BESTIMMUNG VON 6-PHOSPHOGLUCONAT IN BIOLOGISCHEM MATERIAL. Biochem Z. 1964 Aug 11;340:345–350. [PubMed] [Google Scholar]
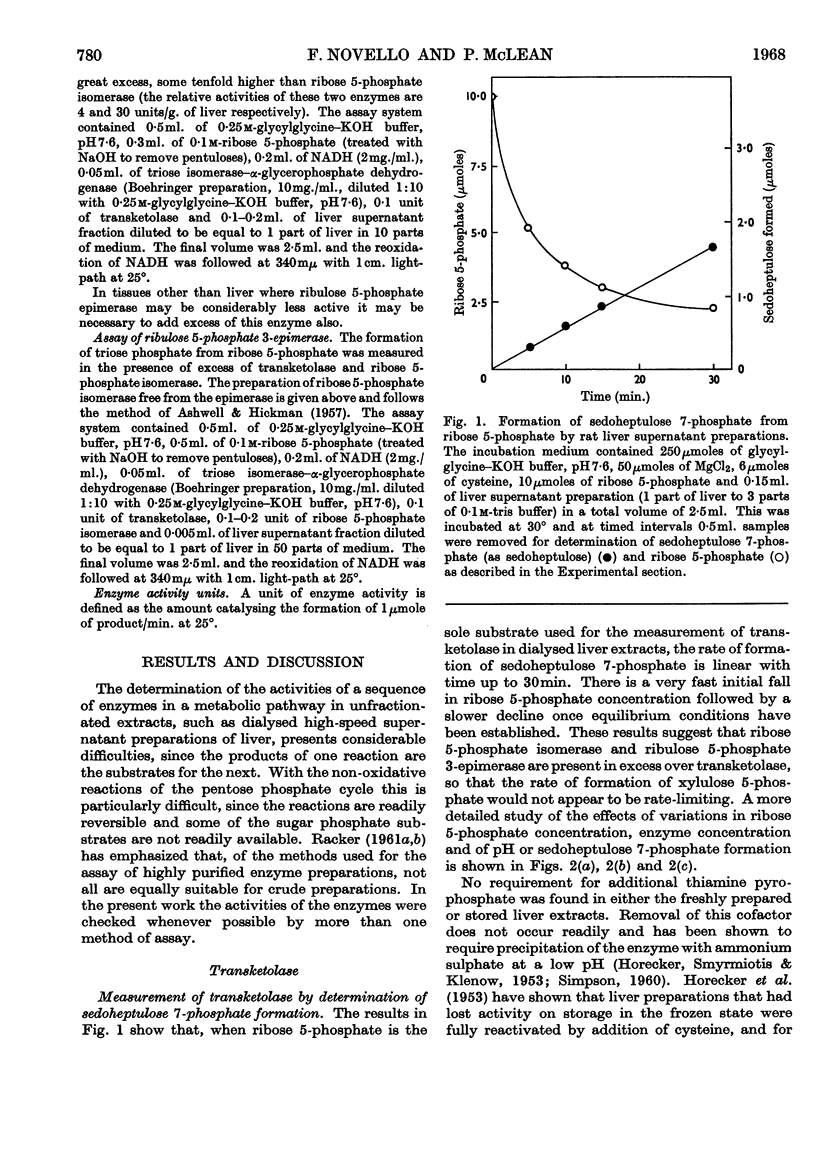
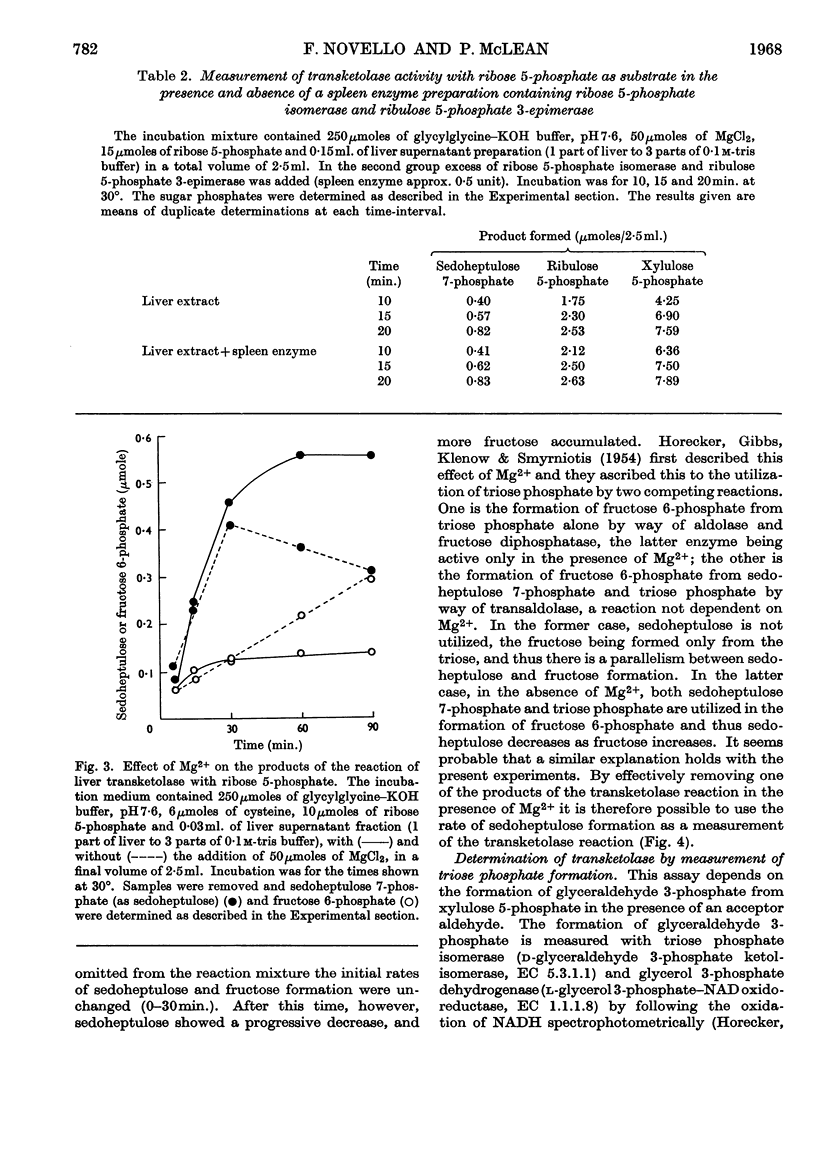
- ASHWELL G., HICKMAN J. Enzymatic formation of xylulose 5-phosphate from ribose 5-phosphate in spleen. J Biol Chem. 1957 May;226(1):65–76. [PubMed] [Google Scholar]
- BAXTER J. N., PERLIN A. S., SIMPSON F. J. Preparation and assay of D-erythrose 4-phosphate. Can J Biochem Physiol. 1959 Feb;37(2):199–209. [PubMed] [Google Scholar]
- BRUNS F. H., NOLTMANN E., VAHLHAUS E. Uber den Stoffwechsel von Ribose-5-phosphat in Hämolysaten. I. Aktivitätsmessung und Eigenschaften der Phosphoribose-isomerase. II. Der Pentosephosphat-Cyclus in roten Blutzellen. Biochem Z. 1958;330(6):483–496. [PubMed] [Google Scholar]
- COLAJACOMO A., MISSALE G., VERGNANO C., LUZZATTO L. Eptoformazione non ossidativa da glucoso-6-fosfato in alcuni tumori sperimentali. Boll Soc Ital Biol Sper. 1957 Oct-Nov;33(10-11):1761–1764. [PubMed] [Google Scholar]
- COOPER J., SRERE P. A., TABACHNICK M., RACKER E. The oxidative pentose phosphate cycle. II. Quantitative determination of intermediates and enzymes. Arch Biochem Biophys. 1958 Apr;74(2):306–314. doi: 10.1016/0003-9861(58)90002-x. [DOI] [PubMed] [Google Scholar]
- DATTA A. G., RACKER E. Mechanism of action of transketolase. I. Properties of the crystalline yeast enzyme. J Biol Chem. 1961 Mar;236:617–623. [PubMed] [Google Scholar]
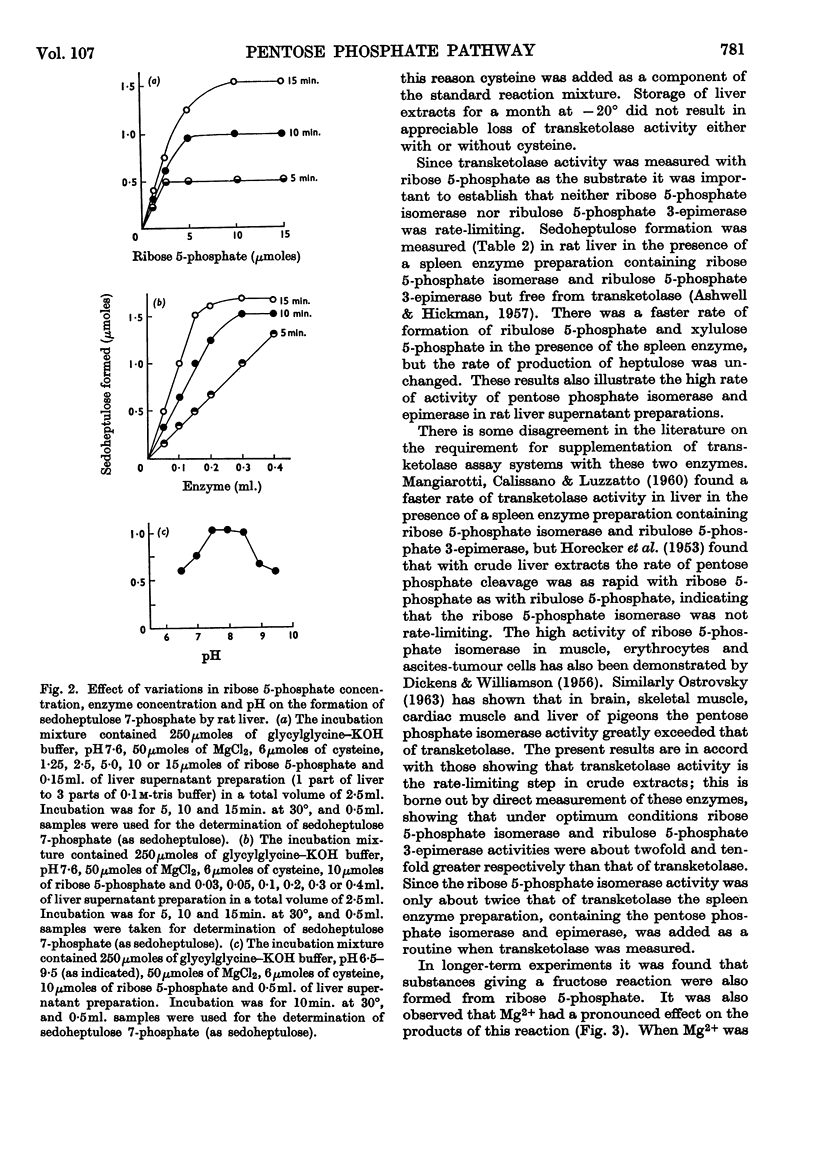
- DICKENS F., WILLIAMSON D. H. Pentose phosphate isomerase and epimerase from animal tissues. Biochem J. 1956 Nov;64(3):567–578. doi: 10.1042/bj0640567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- DISCHE Z., DISCHE M. R. Methods for the qualitative and quantitative determinations of tetroses by two new specific color reactions. Biochim Biophys Acta. 1958 Jan;27(1):184–188. doi: 10.1016/0006-3002(58)90307-x. [DOI] [PubMed] [Google Scholar]
- DISCHE Z., IGALS D. Mechanisms in the interconversion of ribose 5-phosphate and hexose 6-phosphate in human hemolyzates. II. Erythrose 4-phosphate as intermediate and rate regulator in the interconversion of ribose 5-phosphate and hexose 6-phosphate. Arch Biochem Biophys. 1961 May;93:201–210. doi: 10.1016/0003-9861(61)90250-8. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- FEIGELSON P., MARKS P. A. Biosynthesis of liver and tumor RNA ribose in the tumor-bearing rat. Proc Soc Exp Biol Med. 1957 Jun;95(2):376–377. doi: 10.3181/00379727-95-23227. [DOI] [PubMed] [Google Scholar]
- GLOCK G. E., MCLEAN P. A preliminary investigation of the hormonal control of the hexose monophosphate oxidative pathway. Biochem J. 1955 Nov;61(3):390–397. doi: 10.1042/bj0610390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Levels of enzymes of the direct oxidative pathway of carbohydrate metabolism in mammalian tissues and tumours. Biochem J. 1954 Jan;56(1):171–175. doi: 10.1042/bj0560171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E. The formation and breakdown of pentophosphates by liver fractions. Biochem J. 1952 Dec;52(4):575–583. doi: 10.1042/bj0520575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIATT H. H. Studies of ribose metabolism. II. A method for the study of ribose synthesis in vivo. J Biol Chem. 1957 Dec;229(2):725–730. [PubMed] [Google Scholar]
- HORECKER B. L., HURWITZ J., SMYRNIOTIS P. Z. The role of xylulose 5-phosphate in the transketolase reaction. J Biol Chem. 1956 Dec;223(2):1009–1019. [PubMed] [Google Scholar]
- HORECKER B. L., SMYRNIOTIS P. Z., HIATT H. H., MARKS P. A. Tetrose phosphate and the formation of sedoheptulose diphosphate. J Biol Chem. 1955 Feb;212(2):827–836. [PubMed] [Google Scholar]
- HORECKER B. L., SMYRNIOTIS P. Z., KLENOW H. The formation of sedoheptulose phosphate. J Biol Chem. 1953 Dec;205(2):661–682. [PubMed] [Google Scholar]
- MANGIAROTTI G., CALISSANO P., LUZZATTO L. [Transketolase activity in some transplantable tumors in mice]. Boll Soc Ital Biol Sper. 1960 Nov 30;36:1221–1224. [PubMed] [Google Scholar]
- NIGAM V. N., SIE H. G., FISHMAN W. H. Distribution in nature of heptulose-phosphate-forming systems. Can J Biochem Physiol. 1961 Sep;39:1367–1372. doi: 10.1139/o61-145. [DOI] [PubMed] [Google Scholar]
- NIGAM V. N., SIE H. G., FISHMAN W. H. The natural occurrence of sedoheptulose monophosphate in liver. J Biol Chem. 1959 Aug;234(8):1955–1957. [PubMed] [Google Scholar]
- OSTROVSKII Iu M. [The formation of sedoheptulose-7-phosphate in the presence of different states of thiamine supply of the organism]. Biokhimiia. 1963;28:22–30. [PubMed] [Google Scholar]
- PEETERS G., DEBACKERE M. Le cycle oxydatif des hexose-monophosphates dans le tissu mammaire. Arch Int Physiol Biochim. 1956 Jun;64(3):527–528. [PubMed] [Google Scholar]
- PETTE D., KLINGENBERG M., BUECHER T. Comparable and specific proportions in the mitochondrial enzyme activity pattern. Biochem Biophys Res Commun. 1962 Jun 4;7:425–429. doi: 10.1016/0006-291x(62)90328-5. [DOI] [PubMed] [Google Scholar]
- PETTE D., LUH W., BUECHER T. A constant-proportion group in the enzyme activity pattern of the Embden-Meyerhof chain. Biochem Biophys Res Commun. 1962 Jun 4;7:419–424. doi: 10.1016/0006-291x(62)90327-3. [DOI] [PubMed] [Google Scholar]
- SIMPSON F. J. Preparation and properties of transketolase from pork liver. Can J Biochem Physiol. 1960 Feb;38:115–124. [PubMed] [Google Scholar]
- Srivastava L. M., Hübscher G. Glucose metabolism in the mucosa of the small intestine. Enzymes of the pentose phosphate pathway. Biochem J. 1966 Oct;101(1):48–55. doi: 10.1042/bj1010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABACHNICK M., SRERE P. A., COOPER J., RACKER E. The oxidative pentose phosphate cycle. III. The interconversion of ribose 5-phosphate, ribulose 5-phosphate and xylulose 5-phosphate. Arch Biochem Biophys. 1958 Apr;74(2):315–325. doi: 10.1016/0003-9861(58)90003-1. [DOI] [PubMed] [Google Scholar]
- TEPPERMAN H. M., TEPPERMAN J. ON THE RESPONSE OF HEPATIC GLUCOSE-6-PHOSPHATE DEHYDROGENASE ACTIVITY TO CHANGES IN DIET COMPOSITION AND FOOD INTAKE PATTERN. Adv Enzyme Regul. 1963;1:121–136. doi: 10.1016/0065-2571(63)90013-x. [DOI] [PubMed] [Google Scholar]
- VENKATARAMAN R., RACKER E. Mechanism of action of transaldolase. I. Crystalization and properties of yeast enzyme. J Biol Chem. 1961 Jul;236:1876–1882. [PubMed] [Google Scholar]
- WEBER G., BANERJEE G., ASHMORE J. Activities of enzymes involved in glycolysis, glucogenesis and hexosemonophosphate shunt in rat adipose tissue. Biochem Biophys Res Commun. 1960 Aug;3:182–186. doi: 10.1016/0006-291x(60)90219-9. [DOI] [PubMed] [Google Scholar]


