Abstract
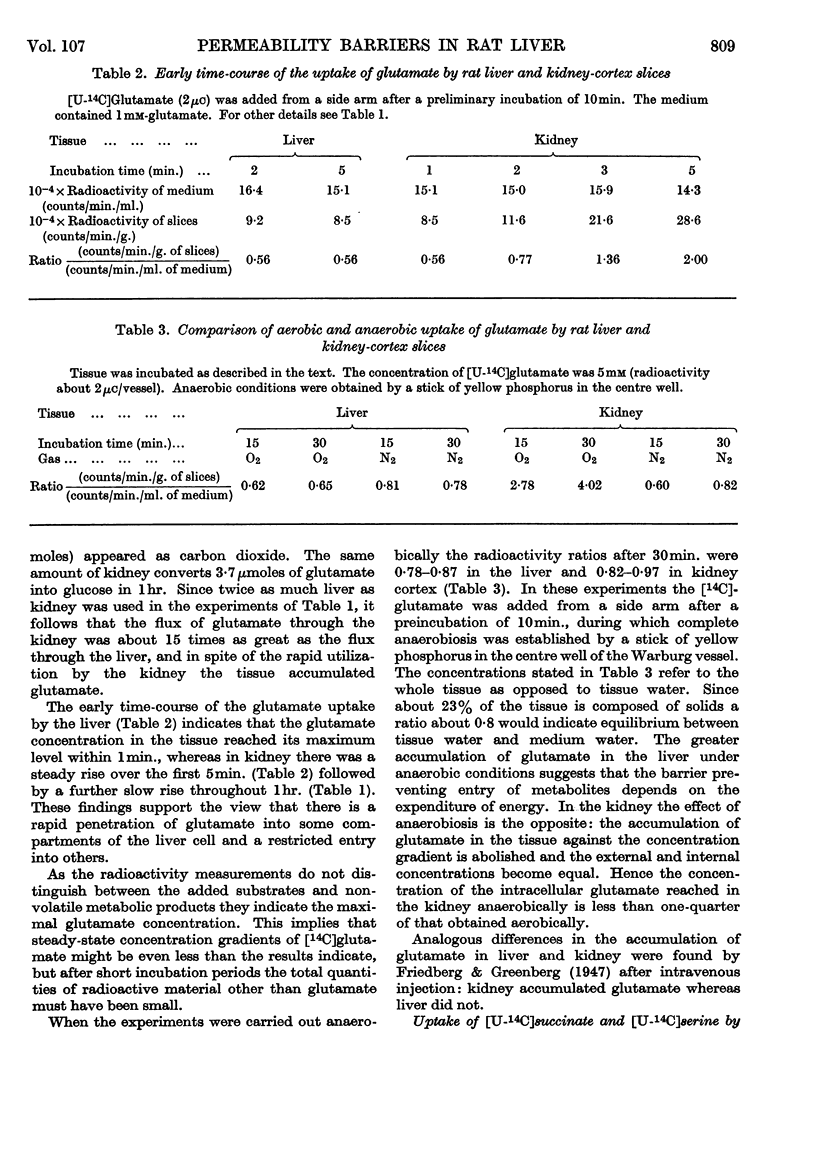
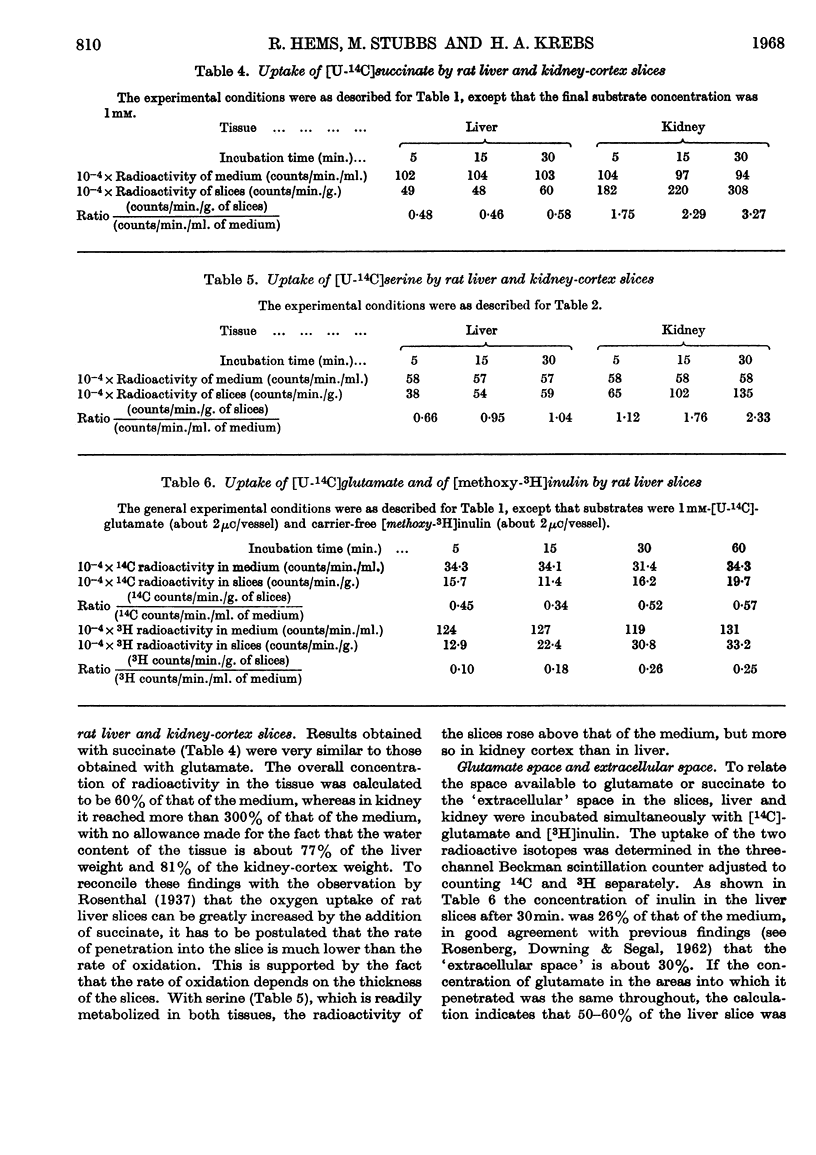
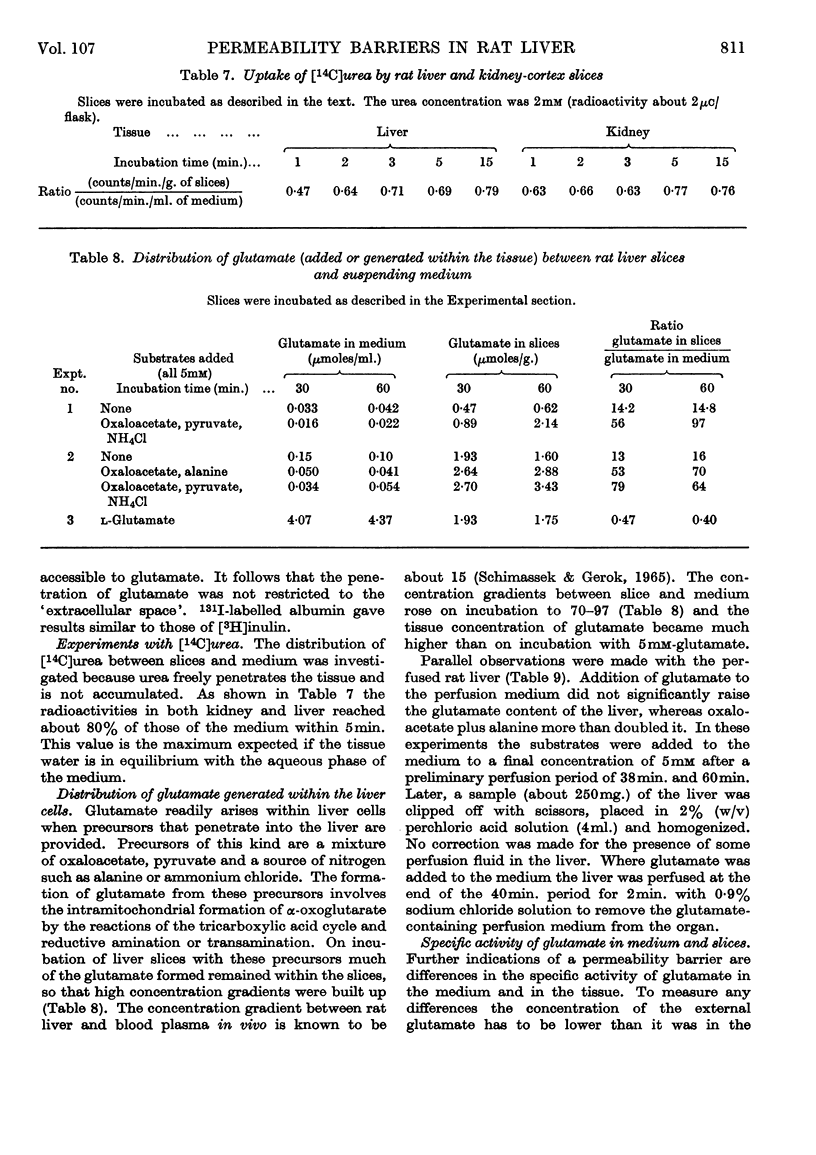
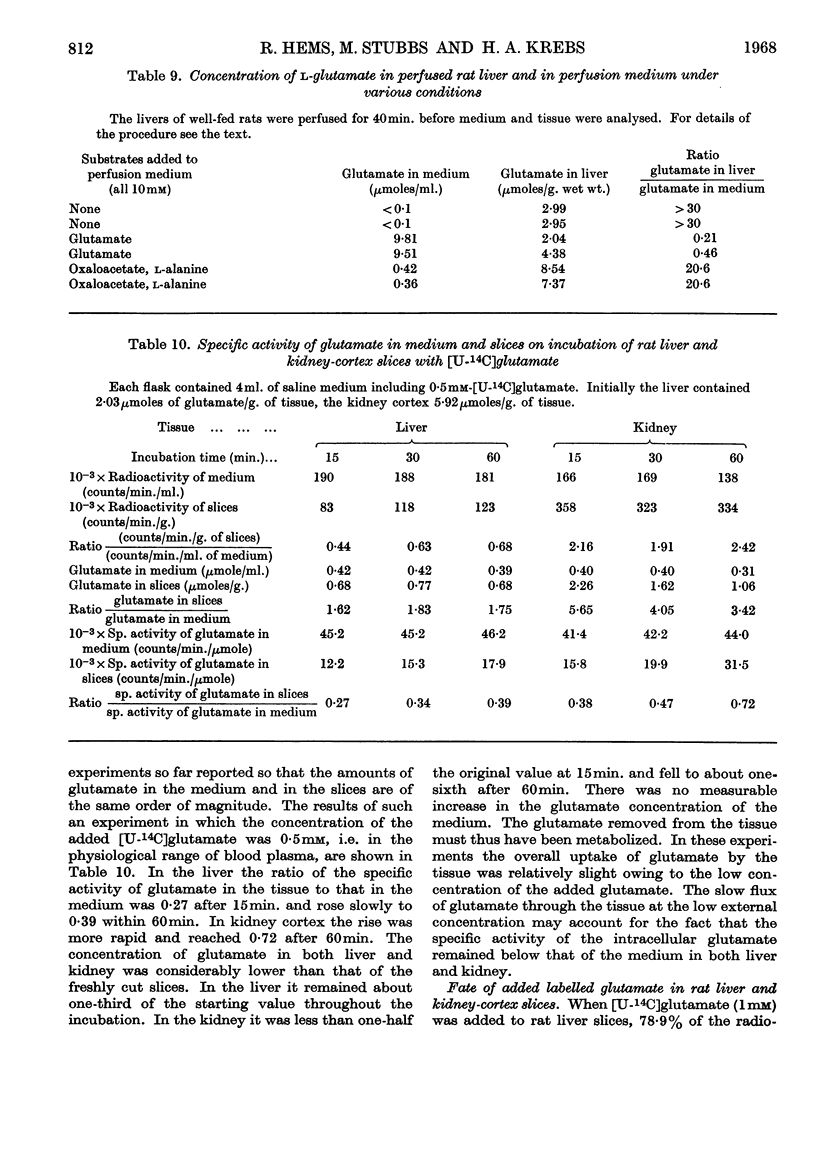
1. When rat liver slices were incubated aerobically with [U-14C]glutamate the concentration of 14C within the slices remained lower (about 50%) than in the medium. The maximal concentration of 14C in the liver was reached within minutes. In rat kidney-cortex slices by contrast, 14C reached concentrations more than six times those of the medium. 2. In both liver and kidney 14C appeared in the respiratory CO2, indicating penetration of glutamate carbon into the mitochondria. In kidney slices the rate of glutamate oxidation per unit weight was about five times that in liver slices. 3. Taking into account the conversion of glutamate into glucose that occurs in the kidney but not in the liver, the flux rates of glutamate through the kidney were calculated to be about 15 times those through the liver when the external glutamate concentration was 5mm. 4. Anaerobically the glutamate concentrations in medium and tissue rapidly became equal in both liver and kidney. Thus the maintenance of concentration gradients depended on the expenditure of energy. 5. [U-14C]Succinate behaved similarly to glutamate. [U-14C]Serine was taken up more rapidly by the kidney than by the liver slices, but the concentrations reached in the liver did not remain below those of the medium. [14C]Urea was distributed evenly between medium and tissue water. 6. Incubation of liver slices with [3H]inulin indicated an extracellular space of liver slices of 26%. 7. When glutamate was generated within liver slices or the perfused liver on addition of oxaloacetate, pyruvate and a source of nitrogen, the concentration of glutamate in the tissue after 1hr. was 70–97 times that in the medium. Thus the exit of glutamate from the liver cell, like its entry, is restricted. This is borne out by measurements of the specific activity of extra- and intra-cellular glutamate on addition of [U-14C]glutamate medium. 8. Liver homogenates removed added glutamate and dicarboxylic acids 20–30 times as fast as did the perfused liver. 9. It is concluded that a major permeability barrier restricts the entry and exit through the outer liver cell membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMOORE J. E. The permeability of isolated rat-liver mitochondria at 0 degree to the metabolites pyruvate, succinate, citrate, phosphate, adenosine 5'-phosphate and adenosine triphosphate. Biochem J. 1958 Dec;70(4):718–726. doi: 10.1042/bj0700718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERL S., TAKAGAKI G., CLARKE D. D., WAELSCH H. Metabolic compartments in vivo. Ammonia and glutamic acid metabolism in brain and liver. J Biol Chem. 1962 Aug;237:2562–2569. [PubMed] [Google Scholar]
- CROWLEY G. J., MOSES V., ULLRICH J. A VERSATILE SOLVENT TO REPLACE PHENOL FOR THE PAPER CHROMATOGRAPHY OF RADIOACTIVE INTERMEDIARY METABOLITES. J Chromatogr. 1963 Oct;12:219–228. doi: 10.1016/s0021-9673(01)83673-6. [DOI] [PubMed] [Google Scholar]
- DICKMAN S. R., SPEYER J. F. Factors affecting the activity of mitochondrial and soluble aconitase. J Biol Chem. 1954 Jan;206(1):67–75. [PubMed] [Google Scholar]
- Gevers W., Krebs H. A. The effects of adenine nucleotides on carbohydrate metabolism in pigeon-liver homogenates. Biochem J. 1966 Mar;98(3):720–735. doi: 10.1042/bj0980720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGEBOOM G. H., SCHNEIDER W. C., STRIEBICH M. J. Cytochemical studies. VII. Localization of endogenous citrate in rat liver fractions. J Biol Chem. 1956 Oct;222(2):969–977. [PubMed] [Google Scholar]
- Harris E. J., van Dam K., Pressman B. C. Dependence of uptake of succinate by mitochondria on energy and its relation to potassium retention. Nature. 1967 Mar 18;213(5081):1126–1127. doi: 10.1038/2131126a0. [DOI] [PubMed] [Google Scholar]
- Haslam J. M., Krebs H. A. The permeability of mitochondria to oxaloacetate and malate. Biochem J. 1968 May;107(5):659–667. doi: 10.1042/bj1070659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., De Gasquet P. Inhibition of gluconeogenesis by alpha-oxo acids. Biochem J. 1964 Jan;90(1):149–154. doi: 10.1042/bj0900149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Eggleston L. V., Hems R. Synthesis of glutamic acid in animal tissues. Biochem J. 1948;43(3):406–414. doi: 10.1042/bj0430406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiitsutsuji-Uwo J. M., Ross B. D., Krebs H. A. Metabolic activities of the isolated perfused rat kidney. Biochem J. 1967 Jun;103(3):852–862. doi: 10.1042/bj1030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS R. A. Lethal synthesis. Proc R Soc Lond B Biol Sci. 1952 Feb 28;139(895):143–170. doi: 10.1098/rspb.1952.0001. [DOI] [PubMed] [Google Scholar]
- ROSENBERG L. E., DOWNING S. J., SEGAL S. Extracellular space estimation in rat kidney slices using C saccharides and phlorizin. Am J Physiol. 1962 Apr;202:800–804. doi: 10.1152/ajplegacy.1962.202.4.800. [DOI] [PubMed] [Google Scholar]
- Rosenthal O. The intensity of succinate oxidation in surviving liver tissue. Biochem J. 1937 Oct;31(10):1710–1718. doi: 10.1042/bj0311710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. D., Hems R., Krebs H. A. The rate of gluconeogenesis from various precursors in the perfused rat liver. Biochem J. 1967 Mar;102(3):942–951. doi: 10.1042/bj1020942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B. Intermediary metabolism of L-cysteinesulfinic acid in animal tissues. Arch Biochem Biophys. 1956 Apr;61(2):397–409. doi: 10.1016/0003-9861(56)90363-0. [DOI] [PubMed] [Google Scholar]
- Schimassek H., Gerok W. Control of the levels of free amino acids in plasma by the liver. Biochem Z. 1965 Dec 31;343(4):407–415. [PubMed] [Google Scholar]
- Schmidt E., Schmidt F. W. Release of enzymes from the liver. Nature. 1967 Mar 18;213(5081):1125–1126. doi: 10.1038/2131125a0. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lopes-Vieira O., Walker B. Concentrations of free glucogenic amino acids in livers of rats subjected to various metabolic stresses. Biochem J. 1967 Aug;104(2):497–502. doi: 10.1042/bj1040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den BERGH S., SLATER E. C. The respiratory activity and permeability of housefly sarcosomes. Biochem J. 1962 Feb;82:362–371. doi: 10.1042/bj0820362. [DOI] [PMC free article] [PubMed] [Google Scholar]



