Abstract
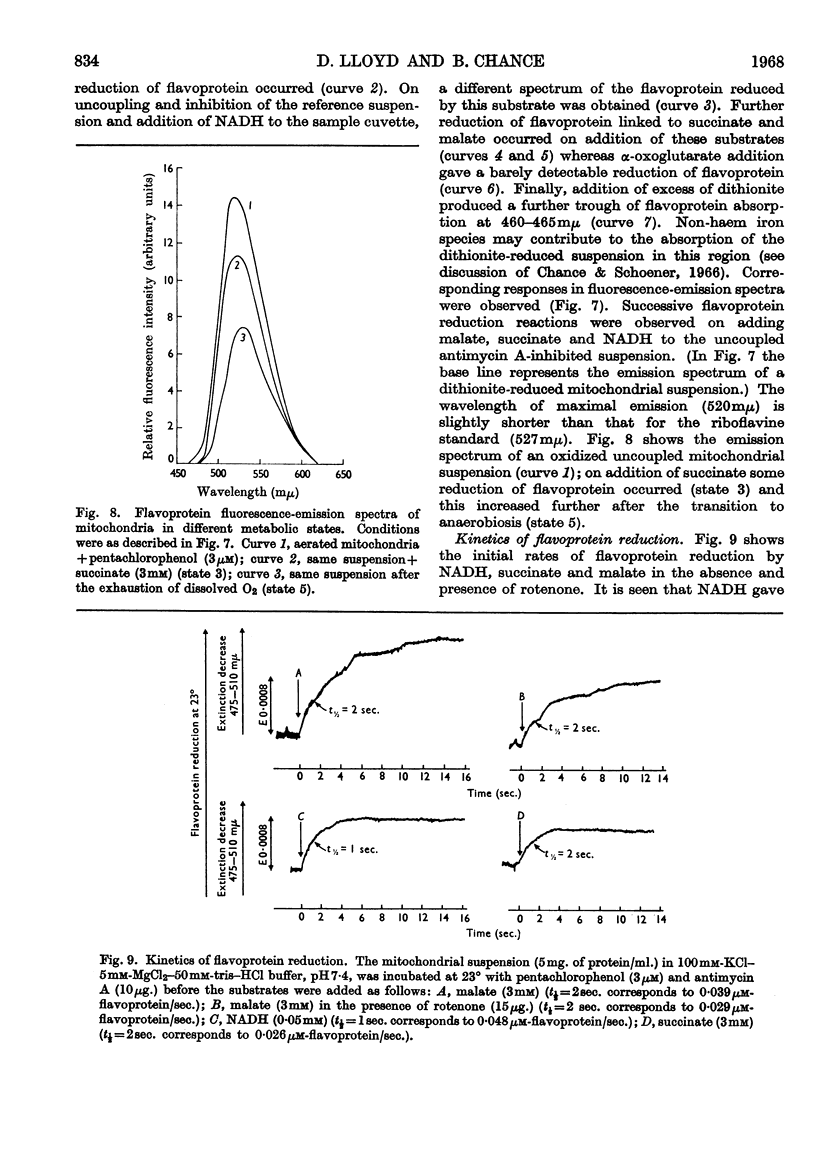
1. Mitochondria isolated from Polytomella caeca contain cytochromes b, c+c1 and a+a3 and several flavoprotein species. 2. Electron transport is inhibited by antimycin A, rotenone, piericidin A and cyanide. 3. Spectral data indicate that antimycin A inhibits the reoxidation of reduced cytochrome b. 4. Various types of flavoprotein are characterized by simultaneous spectrophotometric and fluorimetric measurements on antimycin A-inhibited preparations and also by their absorption and fluorescence-emission spectra. 5. The rotenone-sensitive site lies between the two flavoproteins of the respiratory chain, designated FpD1 and FpD2. 6. Other flavoprotein species detected include those involved in the oxidation of succinate and externally added NADH; a large proportion of mitochondrial flavine is reduced by dithionite but not by known respiratory substrates. 7. The kinetics of flavoprotein and cytochrome reactions were studied.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B., WILLIAMS G. R. A method for the localization of sites for oxidative phosphorylation. Nature. 1955 Aug 6;176(4475):250–254. doi: 10.1038/176250a0. [DOI] [PubMed] [Google Scholar]
- Chance B., Ernster L., Garland P. B., Lee C. P., Light P. A., Ohnishi T., Ragan C. I., Wong D. Flavoproteins of the mitochondrial respiratory chain. Proc Natl Acad Sci U S A. 1967 May;57(5):1498–1505. doi: 10.1073/pnas.57.5.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Sager R. Oxygen and Light Induced Oxidations of Cytochrome, Flavoprotein, and Pyridine Nucleotide in a Chlamydomonas Mutant. Plant Physiol. 1957 Nov;32(6):548–561. doi: 10.1104/pp.32.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Chance B., Ernster L., Lee C. P., Wong D. Flavoproteins of mitochondrial fatty acid oxidation. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1696–1702. doi: 10.1073/pnas.58.4.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C., Wu M., Crane F. L., Takahashi H., Tamura S., Folkers K. Piericidin A: a new inhibitor of mitochondrial electron transport. Biochem Biophys Res Commun. 1966 Nov 22;25(4):373–377. doi: 10.1016/0006-291x(66)90214-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi S. Preparation and properties of mitochondria from the ciliated protozoan, Tetrahymena. J Biochem. 1965 Nov;58(5):444–457. doi: 10.1093/oxfordjournals.jbchem.a128224. [DOI] [PubMed] [Google Scholar]
- Lloyd D. Respiratory control in mitochondria isolated from the colourless alga Prototheca zopfii. Biochim Biophys Acta. 1965 Nov 22;110(2):425–426. doi: 10.1016/s0926-6593(65)80050-9. [DOI] [PubMed] [Google Scholar]
- Lloyd D. The purification and properties of malonic semialdehyde oxidative decarboxylase from Prototheca zopfii. Biochem J. 1965 Sep;96(3):766–770. doi: 10.1042/bj0960766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T., Kawaguchi K., Hagihara B. Preparation and some properties of yeast mitochondria. J Biol Chem. 1966 Apr 25;241(8):1797–1806. [PubMed] [Google Scholar]
- Webster D. A., Hackett D. P. Respiratory Chain of Colorless Algae I. Chlorophyta and Euglenophyta. Plant Physiol. 1965 Nov;40(6):1091–1100. doi: 10.1104/pp.40.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]


