Summary
Patients undergoing cardiopulmonary bypass (CPB) often experience neurological complications, but the neurobiological mechanisms remain unclear. This study combined resting-state fMRI, structural MRI, and cognitive testing to examine brain changes in 124 CPB patients and matched controls. Reduced amplitude of low-frequency fluctuation (ALFF) in the bilateral frontoparietal lobes indicated diminished neural activity among patients, and these ALFF values were positively correlated with the degree of executive dysfunction measured by the attention network test. Functional connectivity within the frontoparietal executive control network was weakened. Brain structural analysis revealed cortical thinning in frontoparietal and temporal regions, increased sulcal depth in medial orbitofrontal areas, and reduced gyrification in the insula suggesting long-term morphological impacts. These findings demonstrate CPB-associated functional and structural alterations in brain regions critical for cognition, providing neuroimaging evidence for postoperative dysfunction and potential neuroprotective strategies.
Subject areas: cardiovascular medicine, medical imaging
Graphical abstract

Highlights
-
•
Cardiopulmonary bypass reduces frontoparietal neural activity
-
•
Executive dysfunction correlates with reduced brain activity
-
•
Cardiopulmonary bypass induces cortical thinning and sulcal depth changes
-
•
Brain imaging reveals targets for neuroprotective strategies
Cardiovascular medicine; Medical imaging
Introduction
Cardiopulmonary bypass (CPB) is a standard procedure in certain cardiovascular surgeries, permitting the cessation of the heart while maintaining circulation and oxygenation.1,2 However, it may be linked to transient or permanent neurologic and neurocognitive complications.3 These complications can prolong postoperative recovery times and may exert a lasting impact on their quality of life, making the study of such issues increasingly valued within the scientific community.4,5 The pathophysiological mechanisms underlying CPB are complex and multifactorial, involving cerebral embolization, microvascular injury, inflammation, and oxidative stress.6,7 Notably, the frontal and temporal lobes, which are crucial for higher cognitive functions such as executive control, memory, and language, are considered to be highly susceptible to the effects of CPB.8,9
The frontal lobe plays a critical role in executive functions, which are essential for planning complex cognitive tasks, logical reasoning, problem-solving, and modulating behavioral responses.10,11 The primary functions of the temporal lobe are to process language, form new memories, and participate in emotional experiences.12 After CPB, these key cognitive domains are often affected. Newman’s team found that postoperative patients exhibited reduced activity in brain regions associated with language, memory, and executive functions, which correlated with declines in cognitive performance.13,14 Grigore’s team demonstrated that the metabolite changes in the frontal and temporal lobes, such as reduced N-acetylaspartate (NAA) and increased choline compounds, were closely related to brain injury and dysfunction following CPB.15 Zanatta’s team found that the fractional anisotropy values of white matter in patients after cardiac surgery were reduced, suggesting that white matter injury is a common phenomenon after CPB.16,17 Therefore, the structural and functional integrity of the frontal and temporal lobes is crucial for maintaining cognitive health and understanding the neurological consequences of CPB.
With advancements in medical imaging technology, neuroimaging has become increasingly important in assessing the structural and functional integrity of the frontal and temporal lobes after CPB. Functional MRI (fMRI) allows for the real-time assessment of brain activity and can detect regional changes in brain function before structural changes become apparent. It has been applied in many cognitive-related diseases and can also be used to observe changes in local and whole-brain neural activity intensity in patients after CPB surgery.18,19 The progression of disease can also cause changes in brain structure, such as the reduction of cortical thickness in the frontal and temporal lobes observed in Alzheimer’s patients, indicating atrophy and potential loss of neurons or synaptic connections in these areas.20 Moreover, the integrity of the cerebral cortex is defined not only by its thickness but also by its folding patterns, which are believed to reflect the complexity of neural networks and their functional interactions. Abnormalities in cortical folding, such as reduced gyrification index and increased sulcal depth, have been reported in the frontal lobes of patients with brain atrophy, suggesting that the disease itself may disrupt the normal development and maintenance of these complex structures.21 Therefore, the application of structural MRI can also help to elucidate the impact of CPB on brain morphology.22,23
This study aims to explore the functional and structural brain changes associated with CPB, with a focus on the frontal and temporal lobes. We hypothesize that CPB will lead to significant alterations in the functional activity and structural integrity of these regions, which will correlate with deficits in cognitive performance. By employing a comprehensive neuroimaging approach, including resting-state fMRI and morphometric analysis,24,25 this study seeks to provide a comprehensive understanding of the neurobiological consequences of CPB and to identify potential targets for intervention.26 The findings may have far-reaching implications, as they could not only help elucidate the mechanisms underlying the observed cognitive decline following CPB but also inform the development of neuroprotective strategies to mitigate these effects. By identifying the specific brain regions and cognitive functions most affected by CPB, we can develop targeted interventions to protect or restore these functions, thereby improving patient outcomes and reducing the burden of neurological complications associated with this common surgical procedure.
Results
Demographic and neuropsychological results
The demographic and neuropsychological results of the experimental and control groups are presented in Table 1. There were no statistically significant differences between the groups in terms of gender (p = 1.000), age (p = 0.147), and education level (p = 0.119). Figure 3A shows no significant differences between the groups in alerting and orienting functions, with p values of 0.485 and 0.318, respectively, while the experimental group exhibited a significantly reduced executive control function compared to the control group (p < 0.001).
Table 1.
Demographics and neuropsychological data
| Characteristic | Experimental group | Control group | p value |
|---|---|---|---|
| n | 124 | 124 | |
| Sex (male/female) | 51/73 | 51/73 | 1.000a |
| Age (years) | 54.11 ± 8.83 | 52.52 ± 8.37 | 0.147b |
| Edu (years) | 8.77 ± 3.31 | 9.36 ± 2.67 | 0.119b |
| ANT | |||
| Alerting (ms) | 55.83 ± 5.01 | 50.56 ± 4.54 | 0.485b |
| Orienting (ms) | 72.75 ± 6.53 | 62.10 ± 5.57 | 0.318b |
| Executive control (ms) | 113.41 ± 15.10 | 20.14 ± 8.83 | <0.001b |
Edu, education; ANT, attention network task.
n represents the sample size.
Chi-squared test.
Two independent-sample t test. Data representation: mean ± SD.
Figure 3.
The relationship between regional brain activity and neurocognition
(A) Patients exhibit significantly prolonged reaction times in executive control functions compared to the control group, indicating a decline in the ability to resolve conflicts in responses and make complex decisions.
(B) illustrates that the executive control function of ANT is positively correlated with the brain activity intensity of the bilateral frontal-parietal lobes (r = 0.534, p < 0.001).
ALFF and brain functional network analysis results
The amplitude of low-frequency fluctuation (ALFF) results were shown in Table 2 and Figure 1, which indicate that the experimental group had significantly reduced ALFF values in the bilateral frontal-parietal lobes and the right temporal lobe compared to the control group (false discovery rate [FDR]-corrected, p < 0.05), and the experimental group’s bilateral cerebellum ALFF values were significantly increased compared to the control group (FDR-corrected, p < 0.05). Figure 2 shows that there are differences in the brain functional network connections composed of the left middle frontal gyrus and the left triangular part of the inferior frontal gyrus between the two groups, with a significant reduction in the patients compared to the control group (FDR-corrected, p < 0.001), and this region’s brain functional network is part of the frontoparietal executive control network. Figure 3B demonstrates a positive correlation between the spontaneous neural activity intensity of the bilateral frontal-parietal lobes and the executive control function in the attention network of all subjects (r = 0.534, p < 0.001).
Table 2.
ALFF alterations in patients compared to the control group
| Brain regions | MNI peak point coordinates |
t value | p value | Voxels | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Bilateral frontal-parietal lobes | 0 | −12 | 78 | −4.65 | 0.019 | 200 |
| Left cerebellum posterior lobe | −24 | −63 | −48 | 3.846 | 0.02 | 80 |
| Right cerebellum posterior lobe | 24 | −75 | −42 | 4.23 | 0.037 | 62 |
| Right temporal lobe | 66 | −48 | 18 | −4.38 | 0.041 | 60 |
ALFF, amplitude of low-frequency fluctuation; MNI, Montreal Neurological Institute.
Adjusted by the false discovery rate (FDR) correction, and the significance threshold was set at p = 0.05.
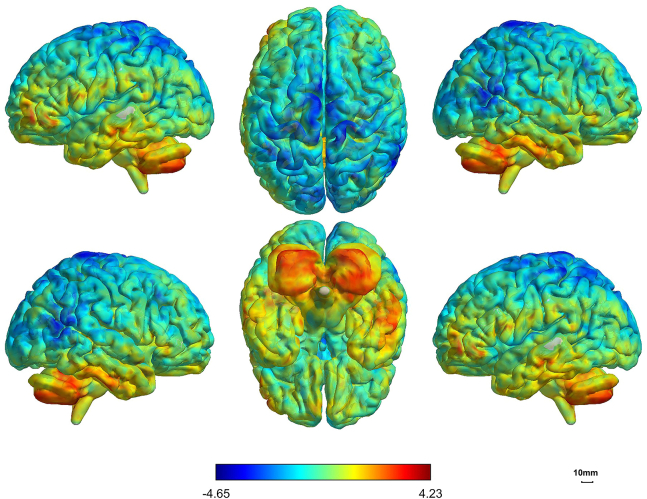
Figure 1.
The alterations in ALFF values between the two groups
The patients exhibited significantly reduced ALFF (amplitude of low-frequency fluctuation) values in the bilateral frontal-parietal lobes and the right temporal lobe compared to the control group (FDR-corrected, p < 0.05) and significantly increased ALFF values in the bilateral cerebellum (FDR-corrected, p < 0.05).
Figure 2.
The differences in brain functional network connectivity between the two groups
The network composed of the left middle frontal gyrus and the left triangular part of the inferior frontal gyrus, with a significant reduction in the patients compared to the control group (FDR-corrected, p < 0.001), and this region’s brain functional network is part of the frontoparietal executive control network.
Brain structural results
Details of cortical thickness changes between the groups are presented in Table 3 and Figure 4A. The experimental group had thinner cortical thickness in the bilateral frontal-parietal lobes and bilateral temporal lobes compared to the control group (FDR-corrected, p < 0.001). The complexity of the cerebral cortex, cortical folding, and sulcal depth structures between the groups are detailed in Table 4 and Figures 4B–4D. The bilateral insula had simpler cortical complexity (FDR-corrected, p < 0.001) and fewer cortical folds (FDR-corrected, p < 0.001) compared to the control group, and the medial orbital frontal lobe and the posterior cingulate gyrus had deeper sulcal depth in the experimental group compared to the control group (FDR-corrected, p = −0.018).
Table 3.
Cortical thickness alterations in the control group compared to patients
| Index and locations | MNI peak point coordinates |
t value | p value | Voxels | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Left frontal-parietal lobe | −5 | 62 | −15 | 9.67 | < 0.001 | 14122 |
| Right frontal-parietal lobe | 24 | 50 | 16 | 9.13 | < 0.001 | 11391 |
| Right temporal lobe | 28 | −51 | −31 | 6.36 | < 0.001 | 509 |
| Left temporal lobe | −28 | −42 | −18 | 6.20 | < 0.001 | 511 |
MNI, Montreal Neurological Institute.
Adjusted by the false discovery rate (FDR) correction, and the significance threshold was set at p = 0.05.
Figure 4.
The alterations in brain structures between the two groups
(A) shows that the patients had thinner cortical thickness in the bilateral frontal-parietal lobes and bilateral temporal lobe compared to the healthy group (FDR-corrected, p < 0.001).
(B) shows that the sulcus depth of the medial orbital frontal lobe and the posterior cingulate gyrus was deeper in patients compared to the control group (FDR-corrected, p = −0.018).
(C) shows that the gyrification index in the bilateral insula was decreased in patients compared to the control group (FDR-corrected, p < 0.001).
(D) shows that the fractal dimension of the bilateral insula was reduced in patients compared to the control group (FDR-corrected, p = 0.001).
Table 4.
Sulcus depth, gyrification index, and fractal dimension alterations in the control group compared to patients
| Index | Overlap of atlas region | p value | Cluster size |
|---|---|---|---|
| Sulcus depth | medial orbitofrontal cortex | −0.018 | 507 |
| caudal anterior cingulate | −0.018 | 308 | |
| Gyrification index | insula | <0.001 | 1002 |
| Insula | <0.001 | 5 | |
| Fractal dimension | Insula | 0.001 | 1002 |
| Insula | 0.001 | 5 |
Adjusted by the false discovery rate (FDR) correction, and the significance threshold was set at p = 0.05.
Discussion
This study utilized resting-state fMRI and structural MRI techniques to examine the brain functional and structural alterations in patients following CPB, with a particular emphasis on the frontoparietal and temporal lobes, which are closely associated with cognitive functions.27,28,29 The study found that CPB patients had significantly reduced ALFF values in the bilateral frontal-parietal and right temporal lobes, while the ALFF values in the cerebellum increased. Furthermore, changes in brain functional networks based on the Power264 template revealed that the CPB patients had significantly reduced connectivity strength in the frontoparietal executive control network compared to the control group. Additionally, cognitive testing showed impaired executive control function in CPB patients, which was positively correlated with ALFF results, further emphasizing the potential negative impact of CPB on higher cognitive functions. On the structural level, CPB patients had significantly reduced cortical thickness in the frontal-parietal and temporal lobes, which may reflect the loss of neurons or synaptic connections. At the same time, we also observed increased sulcal depth in the medial orbital frontal lobe and caudal anterior cingulate and reduced gyrification index and fractal dimension in the insula in the CPB group, which may have long-term effects on cognitive functions. These findings provide detailed neuroimaging characteristics of brain regions affected by CPB and provide important neurobiological evidence for understanding CPB-related cognitive dysfunction.
The frontal-parietal lobes are important in cognitive functions, particularly in executive control.30 This study compared the ALFF values between the CPB patients and control groups and found that CPB patients had reduced ALFF values in the bilateral frontal-parietal lobes, which may reflect reduced neural activity intensity in these brain regions. Research indicates that, during CPB, the patient’s circulation is replaced by an artificial system, which can significantly alter cerebral hemodynamics. This non-physiological circulation may expose brain tissue to the risk of insufficient oxygen and nutrient supply, thereby affecting the normal function of nerve cells.31 The frontoparietal lobes, being distal regions of cerebral blood supply, are particularly susceptible to changes in hemodynamics. This may be one of the underlying mechanisms for the reduced ALFF values observed in the frontoparietal regions following CPB. Moreover, using the widely accepted Power264 template, this study revealed differences in the topological organization of these networks, showing that the CPB patients had significantly lower brain network efficiency in the frontoparietal region compared to the control group, which belongs to the frontoparietal executive control network in the Power264 template.32 This result indicates that the brain networks related to executive control functions in CPB patients were affected, consistent with the ALFF results. Furthermore, the correlation analysis between ALFF values and ANT cognitive functions in this study found that ALFF values in the bilateral frontal-parietal lobes were significantly positively correlated with executive control functions of ANT, and there were significant differences in executive control functions between the CPB patients and control groups, indicating that CPB is associated with a decline in executive functions. Finally, surface-based morphometric analysis (SBM) found that CPB patients had thinner cortical thickness in the bilateral frontal-parietal lobes compared to the control group and deeper sulcal depth in the medial orbital frontal lobe and caudal anterior cingulate, indicating that neurons in the frontal and cingulate regions of the patients may be damaged after CPB, which is consistent with some previous research results.33 In summary, CPB patients exhibited reduced functional activity and thinner cortical thickness in the frontal-parietal lobes and deeper sulcal depth, and these changes in the frontal-parietal lobes were correlated with clinical symptoms—reduced executive control functions, which helps to elucidate the potential mechanisms of executive function decline after CPB and explore possible treatment measures for these secondary neurological complications.34
The temporal lobe plays a crucial role in processing language, memory formation, and emotional regulation. Damage to the temporal lobe is associated with various cognitive dysfunctions, including memory disorders, language disorders, and visual recognition disorders.35 This study showed that CPB patients had reduced ALFF values in the right temporal lobe and thinner cortical thickness in the bilateral temporal lobes, which may reveal the neuro-mechanism of memory decline in patients after CPB. This study also found that the CPB patients had reduced gyrification index and lower fractal dimension in the bilateral insula compared to the control group, which may reflect the loss of neurons and synaptic connections in the insula. These changes may be associated with hemodynamic alterations and changes in blood composition during CPB, which can lead to inflammatory and oxidative stress responses. This is consistent with previous findings that inflammation may affect the functional connectivity of the insula, contributing to the pathophysiology of bipolar disorder.36 In Alzheimer’s disease research, it has been found that changes in the function and structure of the temporal lobe are related to the distribution of dopaminergic, serotonergic, and GABAergic neurotransmitter systems.37 The insula is related to sensory and emotional processing as well as the regulation of the autonomic nervous system.38,39 Damage to the insula may further affect patients’ emotional regulation, pain perception, and autonomic nervous system function, leading to neurological complications after surgery. In addition, studies have shown that there are functional connections among the insula and the frontal-parietal and temporal lobes, and damage to the insula may affect cognitive functions by affecting the network connections of these regions. Therefore, structural changes in the insula may play an important role in neurologic dysfunction after CPB, and more research is needed to clarify its mechanisms and explore possible neuroprotective strategies.40
The cerebellum has traditionally been considered the brain region primarily responsible for coordinating movement and maintaining balance. However, recent studies have shown that the cerebellum is also involved in cognitive functions, particularly attention and executive control processes.41,42 Our study found that CPB patients had increased ALFF values in the cerebellum, which may be related to postoperative motor coordination and cognitive function recovery. In addition, there are extensive fiber connections between the cerebellum and the frontal-parietal and temporal lobes, and damage in these areas may alter cerebellar function through disrupted signal flow.43,44 Therefore, the role of the cerebellum in cognitive dysfunction after CPB should not be overlooked, and further research is needed to explore its potential neuro-mechanisms and therapeutic targets.
In conclusion, this study delved into the effects of CPB on brain structure and function, revealing significant changes in key brain regions post-CPB that are closely associated with cognitive function impairment. The results not only confirm the profound impact of CPB on brain function but also highlight the necessity of understanding the mechanisms behind these changes, which is crucial for developing effective neuroprotective measures. These insights provide a new direction for future research and clinical practice, aiming to optimize surgical procedures, reduce the neurocognitive sequelae of CPB, and thereby enhance the patients' quality of life.
Limitations of the study
This study has several limitations. First, the absence of longitudinal follow-up data prevents assessment of long-term brain changes post-CPB. Second, potential confounding factors such as surgical techniques and CPB parameters (e.g., pump time, temperature) were not analyzed. Third, the range of abnormal brain areas is large and the functional positioning is not very accurate. Future studies should incorporate longitudinal designs, standardized CPB protocols, and advanced imaging techniques to address these limitations.
Resource availability
Lead contact
Requests for further information and resources should be directed to and will be fulfilled by the lead contact, Dr. Dong Zhang (zhangdongxqyy@163.com).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Raw and analyzed data can be acquired from https://github.com/ZD-cloud/raw-and-analyzed-data. They are publicly available as of the date of publication until 2026.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
The authors would like to thank Dr. Yan Yang from the Radiology Department of Chongqing Xinqiao Hospital for her contributions to article collection and the refinement of this manuscript, as well as Chunshui Liang for his help with data collection. This work was supported by the Chongqing Health and Science Joint Youth Fund (2023QNXM036) and the Talent Project of Chongqing (CQYC202103075).
Author contributions
S.Z. and T.L. are the co-first authors; they contributed to the collection of relevant literature and writing the manuscript. Z.W., W.F., W.L., H.Z., and L.W. contributed to the collection of relevant clinical data. D.Z. and Y.W. are the guarantors of this study and had complete access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Raw and analyzed data | This paper | https://github.com/ZD-cloud/raw-and-analyzed-data |
| Software and algorithms | ||
| MATLAB R2022b | MathWorks |
https://www.mathworks.com/products/matlab.html RRID:SCR_001622 |
| DPABI | Yan et al.45 | RRID: SCR_014853; Version:5.1 |
| CAT12 Toolbox | Christian Gaser, Jena University Hospital, Germany |
http://www.neuro.uni-jena.de/cat/; RRID:SCR_007037 |
| Gretna | http://www.nitrc.org/projects/gretna/ | RRID: SCR_007776; Version:2.0.0 |
| Eprime (Attention Network Test) | Psychology Software Tools, Inc.; https://www.pstnet.com/eprime | RRID: SCR_001754; Version:2.0.0 |
| SPSS | IBM Corporation; https://www.ibm.com/analytics/spss-statistics-software | RRID: SCR_002865; Version:22.0 |
Experimental model and study participant Details
Human subjects
Participants
124 CPB patients (51 male/73 female; age 54.11±8.83 years) and 124 controls (51 male/73 female; age 52.52±8.37 years). Data are represented as mean ± SD. Written informed assent was obtained from all participants in this study, as well as written informed consent from their parent or legal guardian.
Gender and race analysis
All the subjects were of Han ethnicity. No significant gender and race differences between groups (p=1.000).
CPB group
87 valvular heart disease and 37 aortic dissection requiring cardiopulmonary bypass under hypothermic conditions; LVEF <50% or LVEDD >70 mm.
Control group
Community-recruited healthy adults, matched for age/sex/education.
Inclusion criteria for the experimental group
Asymptomatic but high-risk valvular heart disease with left ventricular dysfunction (LVEF < 50%) or left ventricular dilation (LVEDD > 70 mm).46 Acute Stanford Type A aortic dissection confirmed by thoracic aorta CTA. Age 35-75 years, right-handed, and with an educational level above primary school. No surgical contraindications found upon clinical evaluation.
Inclusion criteria for the control group
Age 35–75 years, right-handed, and with an educational level above primary school.
All participants underwent screening for the following exclusion criteria
Abnormal brain neuroanatomical structures. Acute psychiatric or behavioral disorders. History of head injury, drug use, or alcohol abuse. Chronic diseases that may affect cognition, such as diabetes, hypertension, rheumatoid arthritis, cancer, or other conditions. Participants with contraindications for MRI imaging or who moved their heads more than 1.5 millimeters or 1.5 degrees during the scanning process were also excluded.47
Method Details
Experimental design
Subjects who underwent cardiopulmonary bypass participated in this experiment after their postoperative condition was stable. All subjects underwent cranial MRI examination (including 8 minutes of resting-state fMRI and thin-section structural images), E-prime cognitive function testing. After data collection, the 124 cardiovascular surgery subjects were finally determined as the experimental group, and 124 subjects were recruited as the control group for intergroup comparison of brain function and structure.
Image acquisition
All subjects underwent resting-state functional magnetic resonance imaging (rs-fMRI) scanning on a 3.0T GE MRI system using a standard 32-channel phased-array head coil. Subjects were instructed to remain quiet and keep their eyes closed during the scanning process. The parameters of this sequence are as follows: number of slices = 45; slice thickness = 3 mm, slice gap = 0 mm; TE (echo time) = 25 ms, TR (repetition time) = 2000 ms, flip angle (FA) = 90°, field of view (FOV) = 240 mm × 240 mm, matrix = 64 × 64, isotropic voxel size = 3 mm × 3 mm × 3 mm, number of time points = 240.
High-resolution brain structural images were obtained using a three-dimensional fast spoiled gradient recalled (3D FSPGR) sequence; the parameters for this sequence were as follows: number of slices = 170; slice thickness = 1.0 mm, slice gap = -0.5 mm; TE = 2.7 ms, TR = 5.9 ms, FA = 15°, FOV = 240 mm × 240 mm, matrix = 256 × 256, isotropic voxel size = 0.5 mm × 0.5 mm × 0.5 mm.
Cognitive testing
All subjects underwent the Attention Network Test (ANT) via the E-prime program, which measures three functions of the attention network: Alerting, Orienting, and Executive Control.48
Image preprocessing
Rs-fMRI Data Processing: Functional image preprocessing was conducted using the DPARSF 5.1 toolbox (http://rfmri.org/dpabi) on the MATLAB (R2022b) platform, with the following steps. 1. Discarding the first 10 time points of functional images. 2. Slice-Timing Correction, ensuring that the timing of the signal is synchronized across the entire brain. 3. Realignment and Motion Correction, the rs-fMRI data are realigned to correct for head motion artifacts, participants with excessive head motion (translations > 1.5 mm or rotations > 1.5°) may be excluded from further analysis. 4. Co-registration, functional images were co-registered to structural images. 5. Segmentation, the anatomical images are segmented into gray matter, white matter and cerebrospinal fluid for further analysis. 6. Spatial normalization, the segmented gray matter is normalized to a standard MNI template for group comparisons. 7. Smoothing, a 6 mm full-width at half-maximum (FWHM) Gaussian kernel was used for spatial smoothing. 8. Temporal filtering, a frequency range of (0.01 Hz < f < 0.08 Hz) was selected. 9. Nuisance variable regression, potential confounding factors such as global signal and signals from white matter and cerebrospinal fluid are regressed out to reduce noise. 10. Detrending was used to remove linear trends.49
Brain Structural MRI Data Preprocessing: Brain structural data preprocessing was conducted using the CAT12 toolbox (SPM12, https://www.fil.ion.ucl.ac.uk/spm) on the MATLAB (R2022b) platform. This study employed the default recommendation process of CAT12 for surface-based morphometry (SBM) processing. The specific steps are as follows. 1. Skull-stripping and bias field correction, before SBM processing, T1-weighted images were skull-stripped to remove non-brain tissues and underwent bias field correction to normalize signal intensity. 2. Tissue segmentation, the brain tissues were segmented into gray matter, white matter, and cerebrospinal fluid. 3. White matter and cortical surface identification, based on voxel intensity information, the boundaries between white matter and gray matter and between gray matter and cerebrospinal fluid were identified. The central surface of the cortex was then generated based on these boundaries. 4. Surface meshing, the white matter surface of each hemisphere was meshed into a triangular grid. 5. Topological correction, topological correction was applied to ensure that the cortical surface topology conformed to biological plausibility and then conduct surface refinement. 6. Spherical inflation and spherical registration, a non-linear registration technique based on folding patterns was used to align each subject’s cortical folding patterns to a standard spherical space. 7. Spherical alignment, individual cortical data on the spherical surface were aligned to a standard space to enable group-level analysis. 8.Surface smoothing, a Gaussian smoothing kernel with a recommended full-width at half-maximum (FWHM) of 15 mm was typically used.50
Amplitude of low-frequency fluctuations (ALFF) and brain network calculation
ALFF was calculated using preprocessed RS-fMRI data to assess the intensity of local spontaneous brain activity in a resting state. For each subject, ALFF of the entire brain was calculated using images preprocessed with band-pass filtering (0.01 Hz < f < 0.08 Hz), which reduces low-frequency drift and high-frequency respiratory and cardiac noise. Finally, the calculated ALFF values were transformed using Fisher's r-to-z transformation and compared between groups.27
Brain functional networks were constructed based on the POWER264 brain network template using graph theoretical methods; node and edge attributes were defined; the clustering coefficient (local connection density) of the network was calculated; and finally, the calculated network properties were compared between groups to identify differences.28
Additional resources
Approved by the Ethics Committee of Xinqiao Hospital (2023-yan NO:166-01,2023). It can be acquired from (https://github.com/ZD-cloud/raw-and-analyzed-data).
Quantification and statistical analysis
Statistical tests: Independent t-tests, chi-square, partial correlations (SPSS 22.0).
Correction: FDR for multiple comparisons (p < 0.05).
Data representation: Mean ± SD, voxel-level thresholds specified. N represents the sample size.
Independent samples t-tests were used in SPSS 22.0 to analyze differences in age, education level, and neuropsychological ANT data between the two groups. The statistical significance threshold was set at p = 0.05 (Table 1).
Chi-square tests were used to analyze differences in gender between the groups by SPSS 22.0. The statistical significance threshold was set at p = 0.05 (Table 1).
In the CAT12 software, two-sample t-tests were applied to investigate differences in ALFF and SBM indices between the experimental and control groups, with gender, age, education level and total intracranial volume serving as covariates. The voxel level threshold was set at p = 0.001, and statistical significance was set at p = 0.05, with FDR correction applied for multiple comparisons across the whole brain (Tables 2, 3, and 4; Figures 1 and 4).
MATLAB was used to compare brain network properties between the two groups, also with FDR correction and a statistical significance threshold of p = 0.05 (Figure 2).
To investigate the relationship between subjects' neuropsychological results and regional brain activity differences, partial correlation analysis was conducted in SPSS 22.0, regressing out gen-der, age, education level and total intracranial volume, with a statistical significance threshold set at p = 0.05 (Figure 3).
Published: March 10, 2025
Contributor Information
Yong Wang, Email: wy003236@163.com.
Dong Zhang, Email: zhangdongxqyy@163.com.
References
- 1.Chernov V.I., Efimova N.Y., Efimova I.Y., Akhmedov S.D., Lishmanov Y.B. Short-term and long-term cognitive function and cerebral perfusion in off-pump and on-pump coronary artery bypass patients. Eur. J. Cardio. Thorac. Surg. 2006;29:74–81. doi: 10.1016/j.ejcts.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Hessel E.A. What's New in Cardiopulmonary Bypass. J. Cardiothorac. Vasc. Anesth. 2019;33:2296–2326. doi: 10.1053/j.jvca.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Brown C.H., Kim A.S., Yanek L., Lewis A., Mandal K., Le L., Tian J., Neufeld K.J., Hogue C., Moghekar A. Association of perioperative plasma concentration of neurofilament light with delirium after cardiac surgery: a nested observational study. Br. J. Anaesth. 2024;132:312–319. doi: 10.1016/j.bja.2023.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen F., Duan Z., Wu Z., Chen Q., Li H. Plasma lipidomics reveals potential lipid markers for the prediction of delayed neurocognitive recovery after cardiac surgery with cardiopulmonary bypass. Clin. Chim. Acta. 2023;548 doi: 10.1016/j.cca.2023.117504. [DOI] [PubMed] [Google Scholar]
- 5.Gilbey T., Milne B., Somer F.D., Kunst G. Neurologic complications after cardiopulmonary bypass - A narrative review. Perfusion. 2022;38:1545–1559. doi: 10.1177/02676591221119312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vedel A.G., Holmgaard F., Danielsen E.R., Langkilde A., Paulson O.B., Ravn H.B., Rasmussen L.S., Nilsson J.C. Blood pressure and brain injury in cardiac surgery: a secondary analysis of a randomized trial. Eur. J. Cardio. Thorac. Surg. 2020;58:1035–1044. doi: 10.1093/ejcts/ezaa216. [DOI] [PubMed] [Google Scholar]
- 7.Wan Z., Li Y., Ye H., Zi Y., Zhang G., Wang X. Plasma S100β and neuron-specific enolase, but not neuroglobin, are associated with early cognitive dysfunction after total arch replacement surgery. Medicine. 2021;100 doi: 10.1097/md.0000000000025446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruggemans E.F. Cognitive dysfunction after cardiac surgery: Pathophysiological mechanisms and preventive strategies. Neth. Heart J. 2013;21:70–73. doi: 10.1007/s12471-012-0347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshimoto A., Inoue T., Morizumi S., Nishi S., Shimizu T., Kurahashi K., Suematsu Y. Analysis of the perioperative change in cognitive function of patients with risk factors for cognitive impairment in cardiovascular surgery. Gen Thorac Cardiovasc Surg. 2019;67:214–218. doi: 10.1007/s11748-018-0999-6. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S., Hu J., Fan W., Liu B., Wen L., Wang G., Gong M., Yang C., Zhang D. Aberrant Cerebral Activity in Early Postmenopausal Women: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Cell. Neurosci. 2018;12 doi: 10.3389/fncel.2018.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badre D., Nee D.E. Frontal Cortex and the Hierarchical Control of Behavior. Trends Cognit. Sci. 2017;22:170–188. doi: 10.1016/j.tics.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung J., Wang X., Wang X., Bi Y. Functional subdivisions in the anterior temporal lobes: a large scale meta-analytic investigation. Neurosci. Biobehav. Rev. 2020;115:134–145. doi: 10.1016/j.neubiorev.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Newman M.F., Kirchner J.L., Phillips-Bute B., Gaver V., Grocott H., Jones R.H., Mark D.B., Reves J.G., Blumenthal J.A., Neurological Outcome Research Group and the Cardiothoracic Anesthesiology Research Endeavors Investigators Longitudinal Assessment of Neurocognitive Function after Coronary-Artery Bypass Surgery. N. Engl. J. Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 14.Newman M.F., Mathew J.P., Grocott H.P., Mackensen G.B., Monk T., Welsh-Bohmer K.A., Blumenthal J.A., Laskowitz D.T., Mark D.B. Central nervous system injury associated with cardiac surgery. Lancet. 2006;368:694–703. doi: 10.1016/S0140-6736(06)69254-4. [DOI] [PubMed] [Google Scholar]
- 15.Grigore A.M., Mathew J., Grocott H.P., Reves J.G., Blumenthal J.A., White W.D., Smith P.K., Jones R.H., Kirchner J.L., Mark D.B., et al. Prospective randomized trial of normothermic versus hypothermic cardiopulmonary bypass on cognitive function after coronary artery bypass graft surgery. Anesthesiology. 2001;95:1110–1119. doi: 10.1097/00000542-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Zanatta P., Forti A., Minniti G., Comin A., Mazzarolo A.P., Chilufya M., Baldanzi F., Bosco E., Sorbara C., Polesel E. Brain Emboli Distribution and Differentiation During Cardiopulmonary Bypass. J. Cardiothorac. Vasc. Anesth. 2013;27:865–875. doi: 10.1053/j.jvca.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Zanatta P., Benvenuti S.M. Monitoring cerebrovascular autoregulation: a potential new tool to prevent neurocognitive complications after cardiac surgery. Minerva Anestesiol. 2018;85:565–567. doi: 10.23736/S0375-9393.18.13093-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z., Bo Q., Li F., Zhao L., Wang Y., Liu R., Chen X., Wang C., Zhou Y. Increased ALFF and functional connectivity of the right striatum in bipolar disorder patients. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;111 doi: 10.1016/j.pnpbp.2020.110140. [DOI] [PubMed] [Google Scholar]
- 19.Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., Vogel A.C., Laumann T.O., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional Network Organization of the Human Brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiter K., Alpert K.I., Cobia D.J., Kwasny M.J., Morris J.C., Csernansky J.C., Wang L. Cognitively normal individuals with AD parents may be at risk for developing aging-related cortical thinning patterns characteristic of AD. Neuroimage. 2012;61:525–532. doi: 10.1016/j.neuroimage.2012.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meregalli V., Alberti F., Madan C.R., Meneguzzo P., Miola A., Trevisan N., Sambataro F., Favaro A., Collantoni E. Cortical complexity estimation using fractal dimension: A systematic review of the literature on clinical and nonclinical samples. Eur. J. Neurosci. 2022;55:1547–1583. doi: 10.1111/ejn.15631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seyedsalehi A., Warrier V., Bethlehem R.A.I., Perry B.I., Burgess S., Murray G.K. Educational attainment, structural brain reserve and Alzheimer’s disease: a Mendelian randomization analysis. Brain. 2023;146:2059–2074. doi: 10.1093/brain/awac392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meuwly E., Feldmann M., Knirsch W., von Rhein M., Payette K., Dave H., Tuura R.O.G., Kottke R., Hagmann C., Latal B., et al. Postoperative brain volumes are associated with one-year neurodevelopmental outcome in children with severe congenital heart disease. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-47328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rove J.Y., Cain M.T., Hoffman J.R., Reece T.B. Noteworthy in Cardiothoracic Surgery 2023. Semin. CardioThorac. Vasc. Anesth. 2024;28:100–105. doi: 10.1177/10892532241246037. [DOI] [PubMed] [Google Scholar]
- 25.von Rhein M., Buchmann A., Hagmann C., Huber R., Klaver P., Knirsch W., Latal B. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain. 2014;137:268–276. doi: 10.1093/brain/awt322. [DOI] [PubMed] [Google Scholar]
- 26.Kok W.F., Koerts J., Tucha O., Scheeren T.W.L., Absalom A.R. Neuronal damage biomarkers in the identification of patients at risk of long-term postoperative cognitive dysfunction after cardiac surgery. Anaesthesia. 2017;72:359–369. doi: 10.1111/anae.13712. [DOI] [PubMed] [Google Scholar]
- 27.Anderson J.S., Nielsen J.A., Froehlich A.L., DuBray M.B., Druzgal T.J., Cariello A.N., Cooperrider J.R., Zielinski B.A., Ravichandran C., Fletcher P.T., et al. Functional connectivity magnetic resonance imaging classification of autism. Brain. 2011;134:3742–3754. doi: 10.1093/brain/awr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown C.H., Probert J., Healy R., Parish M., Nomura Y., Yamaguchi A., Tian J., Zehr K., Mandal K., Kamath V., et al. Cognitive Decline after Delirium in Patients Undergoing Cardiac Surgery. Anesthesiology. 2018;129:406–416. doi: 10.1097/ALN.0000000000002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrison L. Cardiopulmonary Bypass Mean Global Oxygen Delivery May Be Associated with Neurocognitive Preservation during Hypothermic Aortic Surgery. J. Extra Corpor. Technol. 2020;52:289–294. doi: 10.1182/ject-2000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calderon J., Bonnet D., Courtin C., Concordet S., Plumet M.H., Angeard N. Executive function and theory of mind in school-aged children after neonatal corrective cardiac surgery for transposition of the great arteries. Dev. Med. Child Neurol. 2010;52:1139–1144. doi: 10.1111/j.1469-8749.2010.03735.x. [DOI] [PubMed] [Google Scholar]
- 31.Viderman D., Aubakirova M., Nabidollayeva F., Yegembayeva N., Bilotta F., Badenes R., Abdildin Y. Effect of Ketamine on Postoperative Neurocognitive Disorders: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023;12 doi: 10.3390/jcm12134314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guillaume H., Hugues D. Revisiting the Functional Anatomy of the Human Brain: Toward a Meta-Networking Theory of Cerebral Functions. Physiol. Rev. 2020;100:1181–1228. doi: 10.1152/physrev.00033.2019. [DOI] [PubMed] [Google Scholar]
- 33.Riccelli R., Toschi N., Nigro S., Terracciano A., Passamonti L. Surface-based morphometry reveals the neuroanatomical basis of the five-factor model of personality. Soc. Cogn. Affect. Neurosci. 2017;12:671–684. doi: 10.1093/scan/nsw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krause M., Morabito J.E., Mackensen G.B., Perry T.E., Bartels K. Current Neurologic Assessment and Neuroprotective Strategies in Cardiac Anesthesia: A Survey to the Membership of the Society of Cardiovascular Anesthesiologists. Anesth. Analg. 2020;131:518–526. doi: 10.1213/ane.0000000000004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehrler M., Latal B., Kretschmar O., von Rhein M., O'Gorman Tuura R. Altered frontal white matter microstructure is associated with working memory impairments in adolescents with congenital heart disease: A diffusion tensor imaging study. Neuroimage. Clin. 2020;25 doi: 10.1016/j.nicl.2019.102123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen P., Chen F., Chen G., Zhong S., Gong J., Zhong H., Ye T., Tang G., Wang J., Luo Z., et al. Inflammation is associated with decreased functional connectivity of insula in unmedicated bipolar disorder. Brain Behav. Immun. 2020;89:615–622. doi: 10.1016/j.bbi.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Tang X., Guo Z., Chen G., Sun S., Xiao S., Chen P., Tang G., Huang L., Wang Y. A Multimodal Meta-Analytical Evidence of Functional and Structural Brain Abnormalities Across Alzheimer's Disease Spectrum. Ageing Res. Rev. 2024;95 doi: 10.1016/j.arr.2024.102240. [DOI] [PubMed] [Google Scholar]
- 38.Liu T., Deng R., Wang X., Liu P., Xiao Q.X., Liu Q., Zhang Y. Mechanisms of hypoxia in the hippocampal CA3 region in postoperative cognitive dysfunction after cardiopulmonary bypass. J. Cardiothorac. Surg. 2022;17:106–111. doi: 10.1186/s13019-022-01865-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naef N., Ciernik A., Latal B., Liamlahi R., Children’s Heart and Development Research Group Hippocampal volume and cognitive performance in children with congenital heart disease. Pediatr. Res. 2023;94:99–102. doi: 10.1038/s41390-022-02457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esper S.A., Subramaniam K., Tanaka K.A. Pathophysiology of Cardiopulmonary Bypass: Current Strategies for the Prevention and Treatment of Anemia, Coagulopathy, and Organ Dysfunction. Semin. CardioThorac. Vasc. Anesth. 2014;18:161–176. doi: 10.1177/1089253214532375. [DOI] [PubMed] [Google Scholar]
- 41.Buckner R.L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 42.Neukomm A., Ehrler M., Feldmann M., Chaouch A., Knirsch W., Hagmann C., Jakab A., Latal B. Perioperative Course and Socioeconomic Status Predict Long-Term Neurodevelopment Better Than Perioperative Conventional Neuroimaging in Children with Congenital Heart Disease. J. Pediatr. 2022;251:140–148.e3. doi: 10.1016/j.jpeds.2022.07.032. [DOI] [PubMed] [Google Scholar]
- 43.Fontes K., Courtin F., Rohlicek C.V., Saintmartin C., Gilbert G., Easson K., Majnemer A., Marelli A., Chakravarty M.M., Brossardracine M. Characterizing the Subcortical Structures in Youth with Congenital Heart Disease. AJNR. Am. J. Neuroradiol. 2020;41:1503–1508. doi: 10.3174/ajnr.A6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly C., Castellanos F.X. Strengthening Connections: Functional Connectivity and Brain Plasticity. Neuropsychol. Rev. 2014;24:63–76. doi: 10.1007/s11065-014-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan C.G., Wang X.D., Zuo X.N., Zang Y.F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016;14:339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- 46.Zhang S., Fan W., Hu H., Wen L., Gong M., Liu B., Hu J., Li G., Zhang D. Subcortical Volume Changes in Early Menopausal Women and Correlation With Neuropsychological Tests. Front. Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.738679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen Z.Z., Gotlieb N., Erez O., Wiznitzer A., Arbel O., Matas D., Koren L., Henik A. Attentional networks during the menstrual cycle. Behav. Brain Res. 2022;425 doi: 10.1016/j.bbr.2022.113817. [DOI] [PubMed] [Google Scholar]
- 48.Raimondo L., Oliveira Ĺ.A.F., Heij J., Priovoulos N., Kundu P., Leoni R.F., van der Zwaag W. Advances in resting state fMRI acquisitions for functional connectomics. Neuroimage. 2021;243 doi: 10.1016/j.neuroimage.2021.118503. [DOI] [PubMed] [Google Scholar]
- 49.Gorvitovskaia A.Y., Scrimgeour L.A., Potz B.A., Sellke N.C., Ehsan A., Sodha N.R., Sellke F.W. Lower preoperative hematocrit, longer hospital stay, and neurocognitive decline after cardiac surgery. Surgery. 2020;168:147–154. doi: 10.1016/j.surg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang L., Huang G., Ding Q., Liang P., Hu C., Zhang H., Zhan L., Wang Q., Cao Y., Zhang J., et al. Amplitude of low-frequency fluctuation (ALFF) alterations in adults with subthreshold depression after physical exercise: A resting-state fMRI study. J. Affect. Disord. 2021;295:1057–1065. doi: 10.1016/j.jad.2021.08.094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw and analyzed data can be acquired from https://github.com/ZD-cloud/raw-and-analyzed-data. They are publicly available as of the date of publication until 2026.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.