Abstract
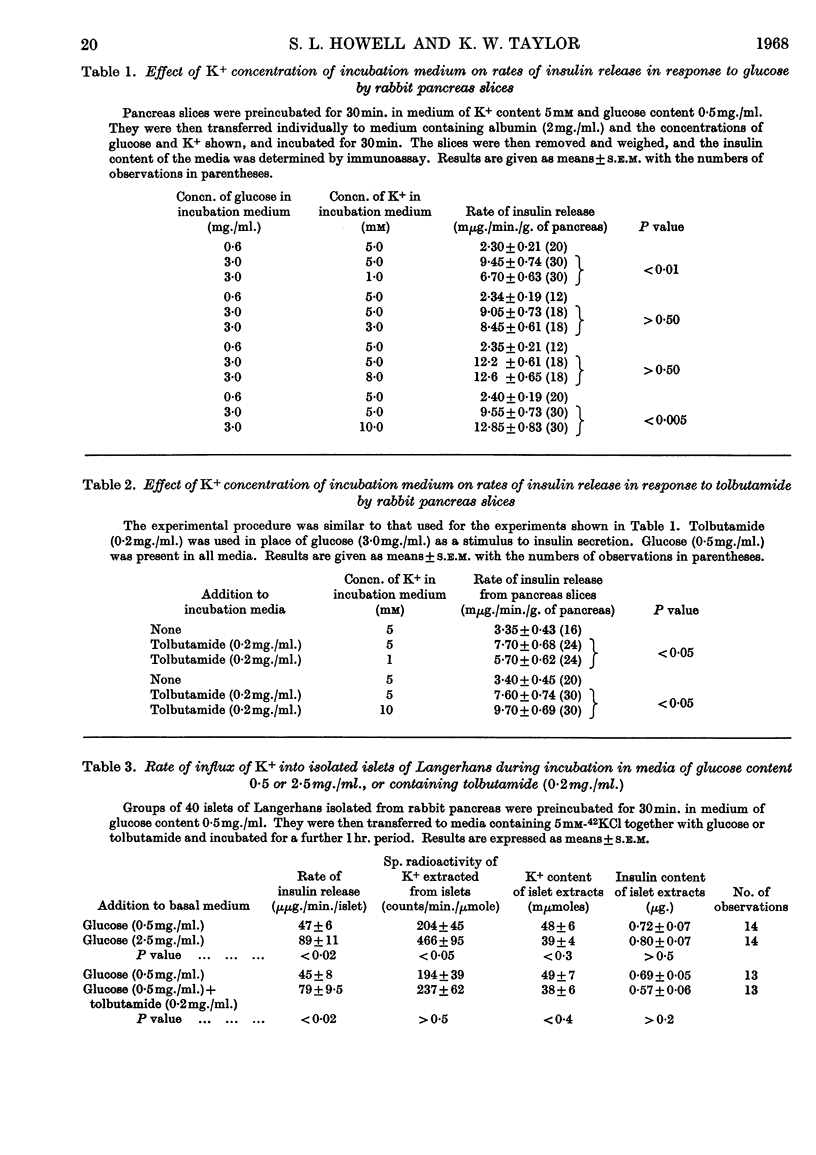
1. A method was devised for the isolation of islets of Langerhans from rabbit pancreas by collagenase digestion in order to study the influx and efflux of K+ in islets during insulin secretion. 2. Glucose-induced insulin release was accompanied by an increased rate of uptake of 42K+ by the islets of Langerhans, though this was not the case for secretion in response to tolbutamide. Ouabain significantly inhibited the uptake of 42K+ by islet tissue. 3. No significant increase in the rate of efflux of 42K+ was demonstrated during active insulin secretion. 4. Slices of rabbit pancreas were incubated in media of different K+ content, and rates of insulin release were determined. Alteration of the K+ concentration of the medium between 3 and 8mm had no effect on the rate of insulin release by pancreas slices. However, decrease of the K+ concentration to 1mm resulted in inhibition of secretion in response to both glucose and to tolbutamide. Conversely, an increase in K+ concentration increased rates of insulin release in response to both these stimuli. 5. It is concluded that, though unphysiological concentrations of K+ may influence the secretion of insulin, fluxes of K+ in the islets do not appear to be important in the initiation of insulin secretion.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BDOLAH A., SCHRAMM M. THE FUNCTION OF 3'5' CYCLIC AMP IN ENZYME SECRETION. Biochem Biophys Res Commun. 1965 Feb 3;18:452–454. doi: 10.1016/0006-291x(65)90730-8. [DOI] [PubMed] [Google Scholar]
- Blond D. M., Whittam R. Effects of sodium and potassium ions on oxidative phosphorylation in relation to respiratory control by a cell-membrane adenosine triphosphatase. Biochem J. 1965 Nov;97(2):523–531. doi: 10.1042/bj0970523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter A. M. Scanning methods: volume quantitation of tissues, cells and subcellular components. J Histochem Cytochem. 1966 Nov;14(11):834–841. doi: 10.1177/14.11.834. [DOI] [PubMed] [Google Scholar]
- Conn J. W. Hypertension, the potassium ion and impaired carbohydrate tolerance. N Engl J Med. 1965 Nov 18;273(21):1135–1143. doi: 10.1056/NEJM196511182732106. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J. 1964 Oct;93(1):66–78. doi: 10.1042/bj0930066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. STIMULUS-SECRETION COUPLING IN A NEUROSECRETORY ORGAN: THE ROLE OF CALCIUM IN THE RELEASE OF VASOPRESSIN FROM THE NEUROHYPOPHYSIS. J Physiol. 1964 Jul;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker S. E. Release of vasopressin and oxytocin from isolated pituitary glands of adult and new-born rats. J Physiol. 1966 Jul;185(2):429–444. doi: 10.1113/jphysiol.1966.sp007994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Effects of acetylcholine and other medullary secretagogues and antagonists on the membrane potential of adrenal chromaffin cells: an analysis employing techniques of tissue culture. J Physiol. 1967 Jan;188(1):107–120. doi: 10.1113/jphysiol.1967.sp008127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol. 1967 Jul;191(1):107–121. doi: 10.1113/jphysiol.1967.sp008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUHRMAN F. A. Glycogen, glucose tolerance and tissue metabolism in potassium-deficient rats. Am J Physiol. 1951 Nov;167(2):314–320. doi: 10.1152/ajplegacy.1951.167.2.314. [DOI] [PubMed] [Google Scholar]
- GARDNER L. I., TALBOT N. B., COOK C. D., BERMAN H., URIBE R. C. The effect of potassium deficiency on carbohydrate metabolism. J Lab Clin Med. 1950 Apr;35(4):592–602. [PubMed] [Google Scholar]
- Grodsky G. M., Bennett L. L. Cation requirements for insulin secretion in the isolated perfused pancreas. Diabetes. 1966 Dec;15(12):910–913. doi: 10.2337/diab.15.12.910. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Effects of glucose concentration on incorporation of [3H]leucine into insulin using isolated mammalian islets of Langerhans. Biochim Biophys Acta. 1966 Dec 28;130(2):519–521. doi: 10.1016/0304-4165(66)90250-9. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. The secretion of newly synthesized insulin in vitro. Biochem J. 1967 Mar;102(3):922–927. doi: 10.1042/bj1020922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACY P. E. Electron microscopy of the beta cell of the pancreas. Am J Med. 1961 Dec;31:851–859. doi: 10.1016/0002-9343(61)90024-9. [DOI] [PubMed] [Google Scholar]
- Lambert A. E., Jeanrenaud B., Renold A. E. Enhancement by caffeine of glucagon-induced and tolbutamide-induced insulin release from isolated foetal pancreatic tissue. Lancet. 1967 Apr 15;1(7494):819–820. doi: 10.1016/s0140-6736(67)92782-1. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Choi S. J. Effects of the cardiac glycosides on the Ca++ uptake of cardiac sarcoplasmic reticulum. J Pharmacol Exp Ther. 1966 Jul;153(1):114–120. [PubMed] [Google Scholar]
- Lundholm L., Rall T., Vamos N. Influence of K-ions and adrenaline on the adenosine 3',-5'-monophosphate content in rat diaphragm. Acta Physiol Scand. 1967 May;70(1):127–128. doi: 10.1111/j.1748-1716.1967.tb03607.x. [DOI] [PubMed] [Google Scholar]
- MCILWAIN H. Phosphates of brain during in vitro metabolism: effects of oxygen, glucose, glutamate, glutamine, and calcium and potassium salts. Biochem J. 1952 Oct;52(2):289–295. doi: 10.1042/bj0520289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSKALEWSKI S. ISOLATION AND CULTURE OF THE ISLETS OF LANGERHANS OF THE GUINEA PIG. Gen Comp Endocrinol. 1965 Jun;5:342–353. doi: 10.1016/0016-6480(65)90059-6. [DOI] [PubMed] [Google Scholar]
- Milner R. D., Hales C. N. The role of calcium and magnesium in insulin secretion from rabbit pancreas studied in vitro. Diabetologia. 1967 Mar;3(1):47–49. doi: 10.1007/BF01269910. [DOI] [PubMed] [Google Scholar]
- Milner R. D., Hales C. N. The sodium pump and insulin secretion. Biochim Biophys Acta. 1967 May 2;135(2):375–377. doi: 10.1016/0005-2736(67)90136-8. [DOI] [PubMed] [Google Scholar]
- Montague W., Howell S. L., Taylor K. W. Pentitols and the mechanism of insulin release. Nature. 1967 Sep 2;215(5105):1088–1089. doi: 10.1038/2151088a0. [DOI] [PubMed] [Google Scholar]
- POST R. L., MERRITT C. R., KINSOLVING C. R., ALBRIGHT C. D. Membrane adenosine triphosphatase as a participant in the active transport of sodium and potassium in the human erythrocyte. J Biol Chem. 1960 Jun;235:1796–1802. [PubMed] [Google Scholar]
- Rolleston F. S., Newsholme E. A. Control of glycolysis in cerebral cortex slices. Biochem J. 1967 Aug;104(2):524–533. doi: 10.1042/bj1040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGILD U., ANDERSEN V., ANDREASEN P. B. Glucose tolerance and insulin responsiveness in experimental potassium depletion. Acta Med Scand. 1961 Mar;169:243–251. doi: 10.1111/j.0954-6820.1961.tb07829.x. [DOI] [PubMed] [Google Scholar]
- Spergel G., Schmidt P., Stern A., Bleicher S. J. Effects of hypokalemia on carbohydrate and lipid metabolism in the rat. Diabetes. 1967 May;16(5):312–318. doi: 10.2337/diab.16.5.312. [DOI] [PubMed] [Google Scholar]
- Sussman K. E., Vaughan G. D. Insulin release after ACTH, glucagon and adenosine-3'-5'-phosphate (cyclic AMP) in the perfused isolated rat pancreas. Diabetes. 1967 Jul;16(7):449–454. doi: 10.2337/diab.16.7.449. [DOI] [PubMed] [Google Scholar]
- Turtle J. R., Littleton G. K., Kipnis D. M. Stimulation of insulin secretion by theophylline. Nature. 1967 Feb 18;213(5077):727–728. doi: 10.1038/213727a0. [DOI] [PubMed] [Google Scholar]
- VOGT M. The secretion of the denervated adrenal medulla of the cat. Br J Pharmacol Chemother. 1952 Jun;7(2):325–330. doi: 10.1111/j.1476-5381.1952.tb01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON J. R., LACY P. E., GRISHAM J. W. Ultrastructural changes in islets of the rat produced by tolbutamide. Diabetes. 1961 Nov-Dec;10:460–469. doi: 10.2337/diab.10.6.460. [DOI] [PubMed] [Google Scholar]
- WOODBURY D. M., WOODBURY J. W. CORRELATION OF MICRO-ELECTRODE POTENTIAL RECORDINGS WITH HISTOLOGY OF RAT AND GUINEA-PIG THYROID GLANDS. J Physiol. 1963 Dec;169:553–567. doi: 10.1113/jphysiol.1963.sp007279. [DOI] [PMC free article] [PubMed] [Google Scholar]


