Abstract
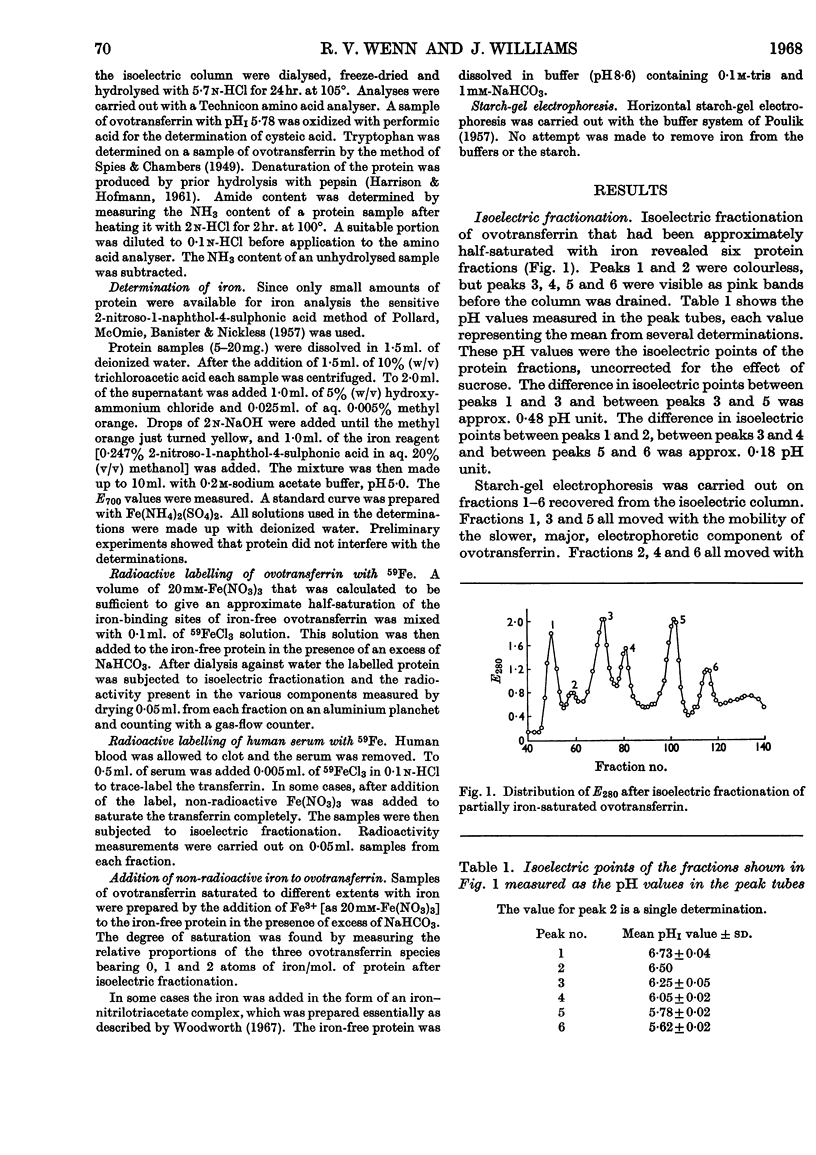
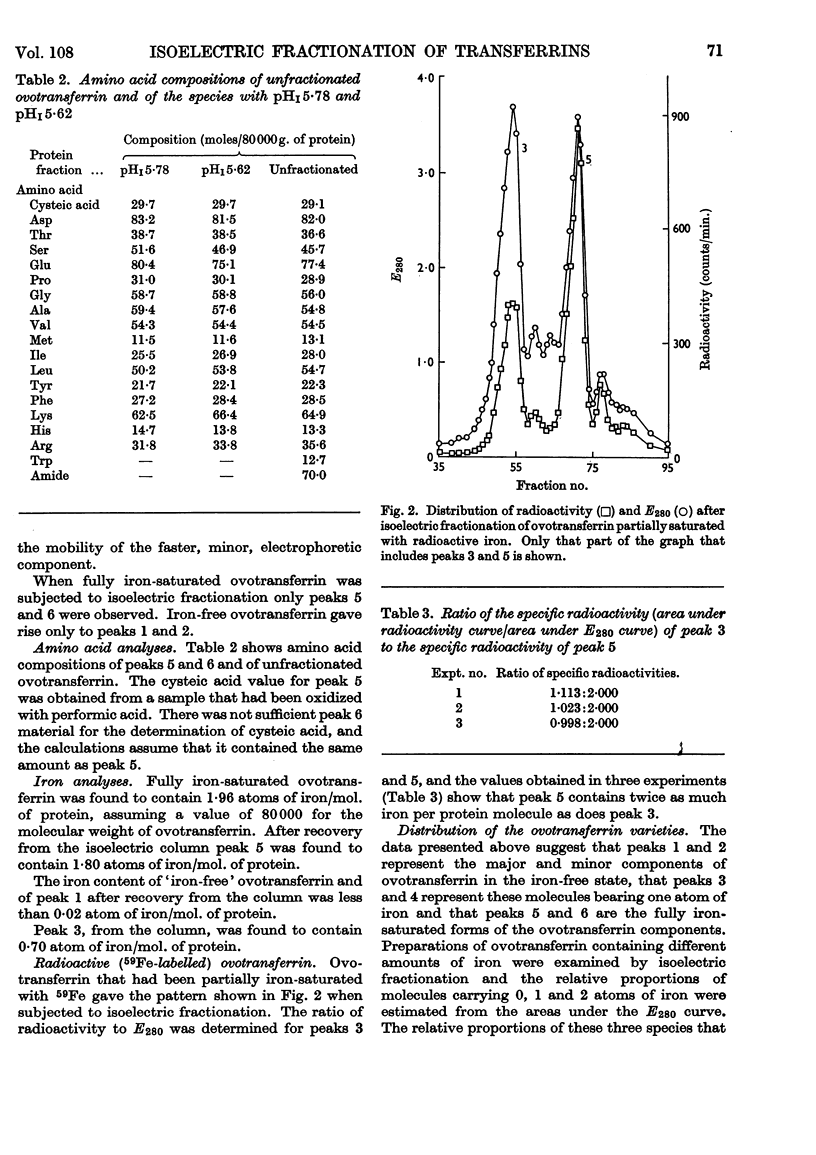
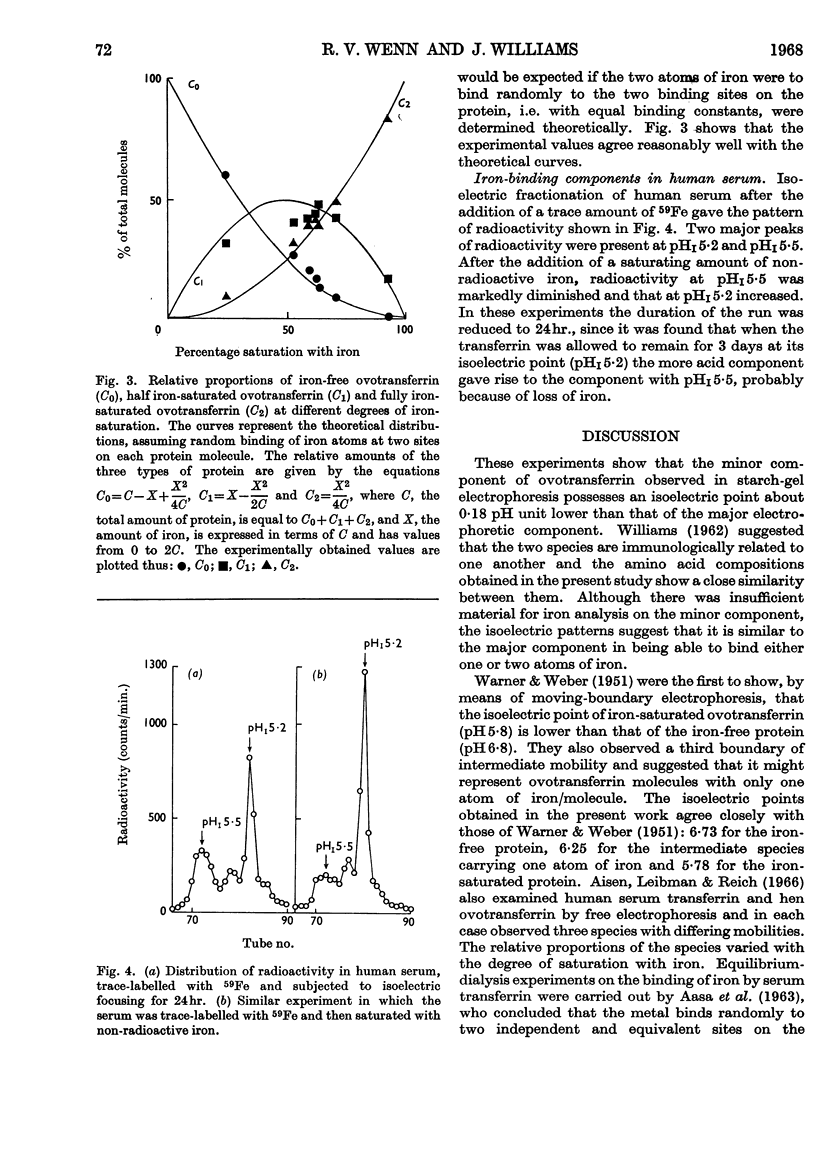
1. Hen ovotransferrin was examined by isoelectric fractionation. 2. The major component observed in starch-gel electrophoresis can be isolated from the minor component. 3. When non-saturating amounts of iron are added to ovotransferrin, isoelectric fractionation demonstrates the existence of three molecular species corresponding to the metal-free protein, the one-iron-atom–protein complex and the two-iron-atoms–protein complex. 4. Isoelectric fractionation of human serum labelled with 59Fe suggests that the transferrin of normal human serum also exists as metal-free protein, the one-iron-atom–protein complex and the two-iron-atoms–protein complex. 5. It is concluded that the binding constants for the first and second iron atoms are similar.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AASA R., MALMSTROEM B. G., SALTMAN P. THE SPECIFIC BINDING OF IRON(III) AND COPPER(II) TO TRANSFERRIN AND CONALBUMIN. Biochim Biophys Acta. 1963 Sep 24;75:203–222. doi: 10.1016/0006-3002(63)90599-7. [DOI] [PubMed] [Google Scholar]
- Aisen P., Leibman A., Reich H. A. Studies on the binding of iron to transferrin and conalbumin. J Biol Chem. 1966 Apr 25;241(8):1666–1671. [PubMed] [Google Scholar]
- Bezkorovainy A. Comparative study of metal-free, iron-saturated and sialic acid-free transferrins. Biochim Biophys Acta. 1966 Oct 31;127(2):535–537. doi: 10.1016/0304-4165(66)90410-7. [DOI] [PubMed] [Google Scholar]
- CLARK J. R., OSUGA D. T., FEENEY R. E. COMPARISON OF AVIAN EGG WHITE CONALBUMINS. J Biol Chem. 1963 Nov;238:3621–3631. [PubMed] [Google Scholar]
- Carlström A., Vesterberg O. Isoelectric focusing and separation of the subcomponents of lactoperoxidase. Acta Chem Scand. 1967;21(1):271–278. doi: 10.3891/acta.chem.scand.21-0271. [DOI] [PubMed] [Google Scholar]
- LUSH I. E. Genetic polymorphisms in the egg albumen proteins of the domestic fowl. Nature. 1961 Mar 25;189:981–984. doi: 10.1038/189981a0. [DOI] [PubMed] [Google Scholar]
- POULIK M. D. Starch gel electrophoresis in a discontinous system of buffers. Nature. 1957 Dec 28;180(4600):1477–1479. doi: 10.1038/1801477a0. [DOI] [PubMed] [Google Scholar]
- Roop W. E., Putnam F. W. Purification and properties of human transferrin C and a slow moving genetic variant. J Biol Chem. 1967 May 25;242(10):2507–2513. [PubMed] [Google Scholar]
- SCHADE A. L. IRON UPTAKE BY ERYTHROPOIETIC AND OTHER TISSUES FROM "NATIVE" SERUM SIDEROPHILIN AND FROM ISOLATED "PURIFIED" SIDEROPHILIN. Farmaco Sci. 1964 Feb;19:185–202. [PubMed] [Google Scholar]
- Stratil A. The effect of iron addition to avian egg white on the behaviour of conalbumin fractions in starch gel electrophoresis. Comp Biochem Physiol. 1967 Jul;22(1):227–233. doi: 10.1016/0010-406x(67)90183-1. [DOI] [PubMed] [Google Scholar]
- WARNER R. C., WEBER I. The preparation of crystalline conalbumin. J Biol Chem. 1951 Jul;191(1):173–180. [PubMed] [Google Scholar]
- WILLIAMS J. Serum proteins and the livetins of hen's-egg yolk. Biochem J. 1962 May;83:346–355. doi: 10.1042/bj0830346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A comparison of glycopeptides from the ovotransferrin and serum transferrin of the hen. Biochem J. 1968 Jun;108(1):57–67. doi: 10.1042/bj1080057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka R., Fujii T., Ito Y. On electrophoretic resolution and densitometric determination of apo-transferrin and iron-bound transferrin. Biochem Biophys Res Commun. 1966 Jul 20;24(2):203–207. doi: 10.1016/0006-291x(66)90720-0. [DOI] [PubMed] [Google Scholar]


