Abstract
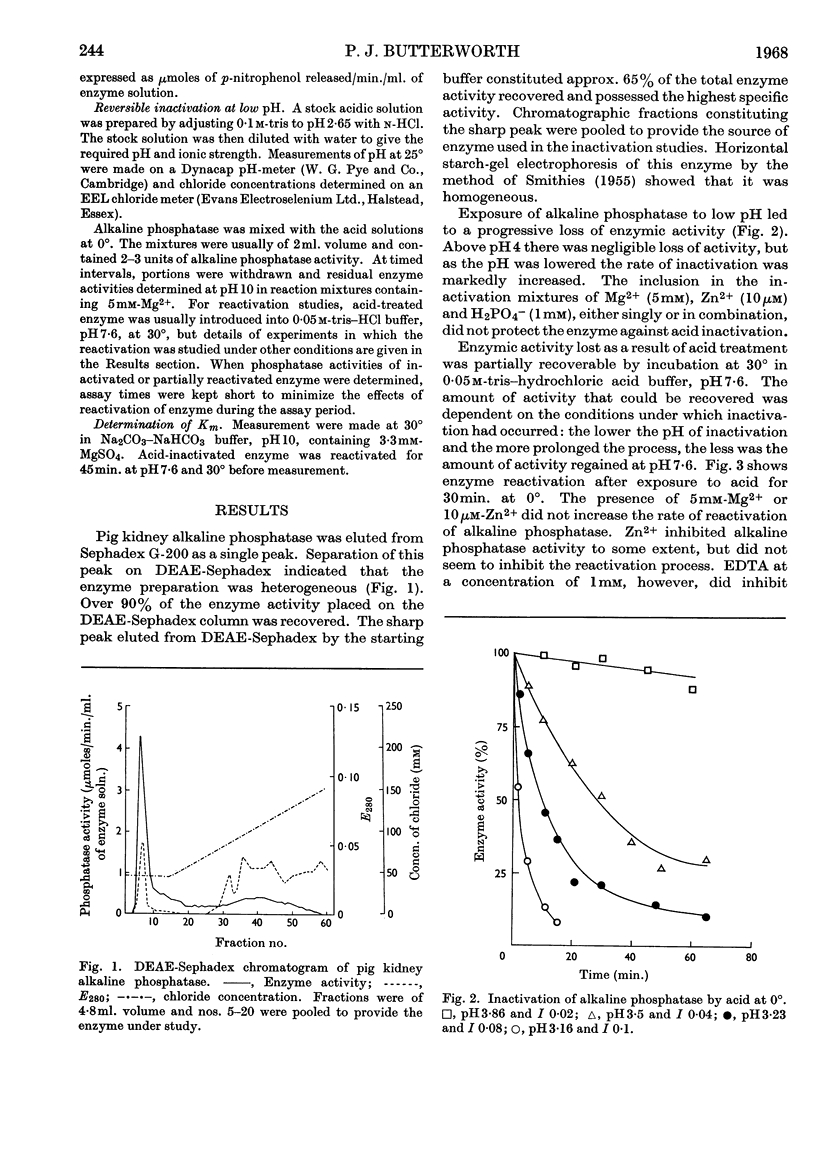
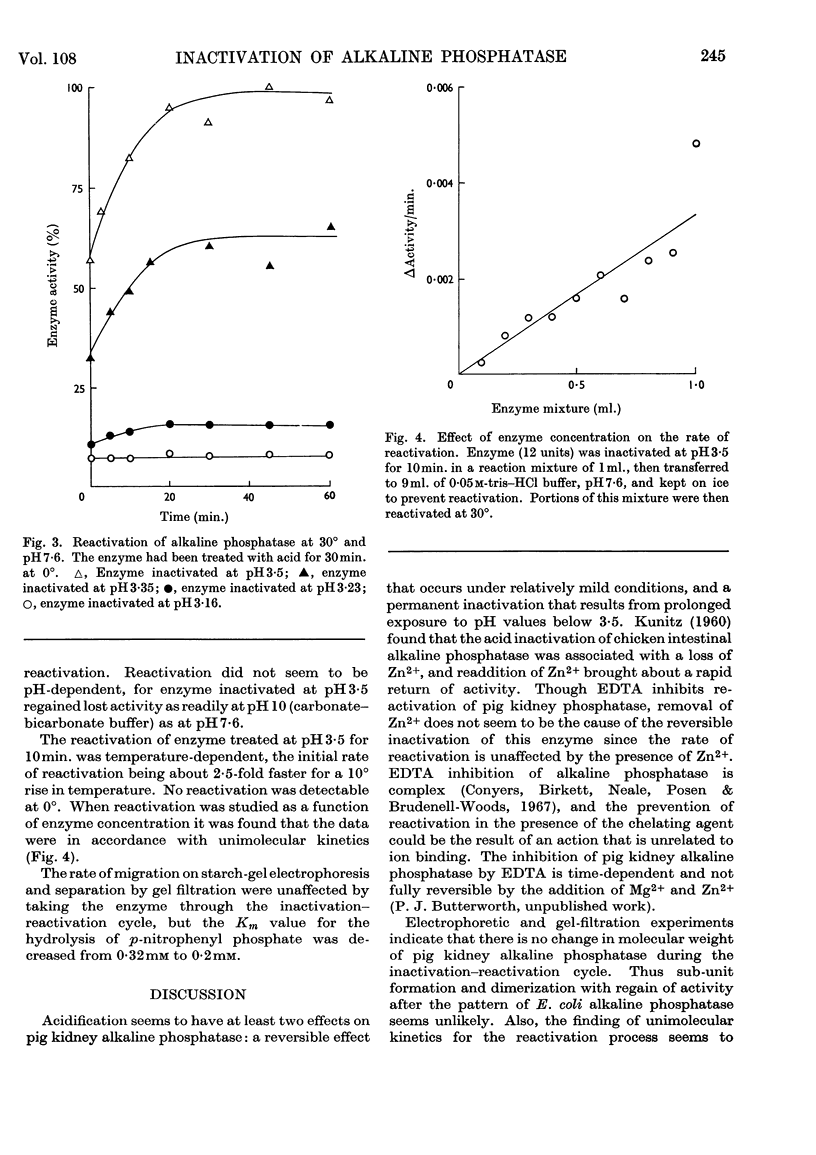
1. Pig kidney alkaline phosphatase is inactivated by treatment with acid at 0°. 2. Inactivated enzyme can be partially reactivated by incubation at 30° in neutral or alkaline buffer. The amount of reactivation that occurs depends on the degree of acid treatment; enzyme that has been inactivated below pH3·3 shows very little reactivation. 3. Studies of the kinetics of reactivation indicate that the process is greatly accelerated by increasing temperature and proceeds by a unimolecular mechanism. The reactivated enzyme has electrophoretic and gel-filtration properties identical with those of non-treated enzyme. 4. The results can be best explained by assuming that a lowering of the pH causes a reversible conformational change of the alkaline phosphatase molecule to a form that is no longer enzymically active but is very susceptible to permanent denaturation by prolonged acid treatment. A reactivation mechanism involving sub-unit recombination seems unlikely.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butterworth P. J. Human kidney and urinary alkaline phosphatases. Biochem J. 1968 Apr;107(4):467–472. doi: 10.1042/bj1070467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conyers R. A., Birkett D. J., Neale F. C., Posen S., Brudenell-Woods J. The action of EDTA on human alkaline phosphatases. Biochim Biophys Acta. 1967 Jul 11;139(2):363–371. doi: 10.1016/0005-2744(67)90040-x. [DOI] [PubMed] [Google Scholar]
- KUNITZ M. Chicken intestinal alkaline phosphatase. I. The kinetics and thermodynamics of reversible inactivation. 2. Reactivation by zinc ions. J Gen Physiol. 1960 Jul;43:1149–1169. doi: 10.1085/jgp.43.6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINTHAL C., SIGNER E. R., FETHEROLF K. Reactivation and hybridization of reduced alkaline phosphatase. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1230–1237. doi: 10.1073/pnas.48.7.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. Separation and purification of enzymes associated with insoluble particles. Nature. 1950 Dec 30;166(4235):1092–1095. doi: 10.1038/1661092a0. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Barrett K. The reversible dissociation of the alkaline phosphatase of Escherichia coli. I. Formation and reactivation of subunits. J Biol Chem. 1965 Nov;240(11):4284–4292. [PubMed] [Google Scholar]


