Abstract
S100B is an astrocytic calcium-binding protein which has been proposed as a biochemical marker of brain damage or dysfunction in acute and chronic diseases. We investigated whether serum S100B levels could be related to systemic lupus erythematosus (SLE) activity. Patients were grouped as having inactive SLE (ISLE), active SLE without central nervous system (CNS) involvement (ASLE), or active SLE with unequivocal neurologic or psychiatric manifestation (NPSLE). The control group consisted of age- and sex-matched healthy blood donors. S100B levels were determined using a luminescence immunoassay. All SLE groups had higher levels of serum S100B than the control group. Among the SLE groups, significantly higher levels of serum S100B protein were found in the NPSLE group than in the ISLE and ASLE groups, and there was no significant difference in S100B levels between the ISLE and ASLE groups. These preliminary results point to a putative relevance of serum S100B protein levels in SLE patients, specifically concerning CNS involvement present in this disease.
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by the production of a variety of autoantibodies, several biochemical abnormalities, and involvement of multiple organ systems (9). The involvement of the central nervous system (CNS) in SLE, causing neuropsychiatric syndromes (NPSLE), is one of the most serious complications of this disease. The NPSLE can vary from mild forms, such as headaches or mild cognitive deficits, to severe states, such as cerebritis, vascular accidents, or psychosis. Recently, a significant positive association of levels of serum antibodies against the specific astrocytic protein glial fibrillary acidic protein with NPSLE was reported (16), suggesting a neural involvement in its pathophysiology.
S100B is a calcium-binding protein expressed and released predominantly by astrocytes (5), and its neurotrophic and gliotrophic actions have been implicated in development and maintenance of the nervous system (2, 4). Furthermore, elevated levels of S100B in cerebrospinal fluid (CSF) and serum could indicate astrocyte activation or blood-brain barrier dysfunction, which allows its study as a marker of CNS injury in some acute and chronic diseases, including brain ischemia (8, 23, 24), brain hemorrhage (3), multiple sclerosis (11), Alzheimer’s disease (2, 17), and schizophrenia (12). Accordingly, we previously have demonstrated that serum S100B levels are elevated in patients with human T-cell leukemia virus type 1 (HTLV-1)-associated myelopathy or tropical spastic paraparesis but not in asymptomatic HTLV-I-positive patients (21). The present work was performed in order to investigate serum S100B levels in SLE patients with and without CNS activity.
MATERIALS AND METHODS
Subjects.
We measured serum S100B levels in 32 SLE patients monitored at the Rheumatology Service of the Hospital de Clínicas de Porto Alegre. All patients fulfilled the American College of Rheumatology (ACR) (1) criteria for SLE. Patients were grouped according to the clinical presentation, laboratory findings, and SLE disease activity index (SLEDAI) as having inactive SLE (ISLE) (n = 13), active SLE without CNS involvement (ASLE) (n = 13), or active NPSLE (n = 6). Neuropsychiatric pathologies (defined according to the ACR nomenclature for NPSLE) were stroke at least 2 weeks before S100B analysis (n = 4), transverse myelitis (n = 1), and chorea (n = 1). None of patients showed signs of hemodynamic instability, infections, or renal or hepatic failure. Fifteen samples from age- and sex-matched healthy blood donors, collected and stored under similar conditions, were used as a control group. This work was approved by local Ethics Committee of Hospital de Clínicas de Porto Alegre, Porto Alegre, Rio Grande do Sul, Brazil. Informed consent was obtained from patients and controls.
Experimental procedure.
Blood samples (5 ml) were collected without anticoagulants by venipuncture. Serum was obtained by centrifugation at 3,000 × g for 5 min and, soon afterwards, kept frozen at −70°C until analysis.
Levels of S100B protein in serum were determined using a luminescence assay (LIAmat; BYK-Sangtec, Broma, Sweden), as previously described (12, 21). The samples were measured in duplicate, and those with a coefficient of variation of above 10% had their measurement repeated.
Statistical analysis.
Comparisons among groups related to age and time course of disease were performed by analysis of variance. Post hoc analysis was performed with the Tukey test when necessary. Comparisons of SLEDAI mean scores among SLE groups and of serum S100B levels among all groups were performed by Kruskal-Wallis analysis of variance followed by a Mann-Whitney test when necessary. A P value of <0.05 was considered statistically significant.
RESULTS
Clinical and demographic data are shown in Table 1. There was no difference in age among groups (P > 0.4) or in the time course of the disease among the SLE groups (P > 0.2). The results show a significant difference in SLEDAI score among SLE groups (P < 0.05); higher values were found in NPSLE patients, followed by ASLE and ISLE patients.
TABLE 1.
Clinical and demographic data
| Characteristic | No. of subjects or value for group
|
|||
|---|---|---|---|---|
| Control (n = 15) | ISLE (n = 13) | ASLE (n = 13) | NPSLE (n = 06) | |
| Sex | ||||
| Female | 10 | 11 | 10 | 4 |
| Male | 5 | 2 | 3 | 2 |
| Race | ||||
| White | 12 | 11 | 12 | 5 |
| Black | 3 | 3 | 1 | 1 |
| Mean age, yr (SD) | 35.6 (8.2) | 40.5 (8.8) | 33.5 (12.4) | 39.6 (15.8) |
| Mean yr of disease (SD) | 7.6 (3.7) | 5.6 (3.1) | 5.4 (2.1) | |
| Mean SLEDAI score (SD) | 1.66 (1.37) | 9.90 (2.88) | 25.0 (7.74) | |
| No. of patients using prednisone (dose, mg/day) | 5 (7) | 13 (35.5) | 6 (60) | |
| No. of patients using cytotoxic drugs | ||||
| Cyclophosphamide | 7 | 4 | ||
| Azathioprine | 9 | |||
| Methotrexate | 1 | |||
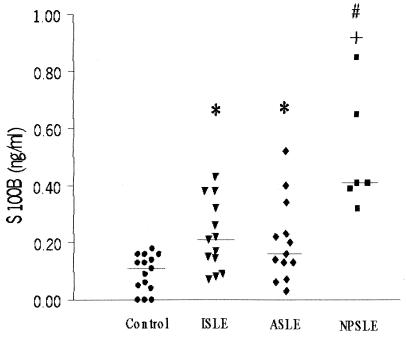
Serum S100B levels in all groups are presented in Fig. 1. There were higher levels (expressed as median [interquartile range]) of serum S100B in the ISLE (0.21 [0.13 to 0.26]) (P < 0.05), ASLE (0.16 [0.12 to 0.26]) (P < 0.05), and NPSLE (0.41 [0.39 to 0.65]) (P < 0.0001) groups than in the control group (0.11 [0.04 to 0.16]). In addition, among SLE patients, serum S100B levels were significantly higher in the NPSLE group than in the ISLE and ASLE groups (P < 0.005), and there was no significant difference in serum S100B levels between the ISLE and ASLE groups (P > 0.5).
FIG. 1.
Serum S100B levels in patients and control groups. Serum S100B levels were significantly higher in NPSLE patients (▪) (median, 0.41 ng/ml) than in ISLE patients (▾) (median, 0.21 ng/ml) and ASLE patients (⧫) (median, 0.16 ng/ml) (P < 0.005 [#]), as well as controls (•) (median, 0.11 ng/ml) (P < 0.0001 [+]). Moreover, the ISLE and ASLE groups showed higher S100B levels than controls (P < 0.05 [*]). Horizontal bars indicate medians.
DISCUSSION
The present work preliminarily demonstrated that serum S100B levels were elevated in all groups of patients with SLE compared to the control group. Furthermore, among SLE patients, serum S100B levels were increased in patients with active SLE with CNS injury compared to SLE patients without brain dysfunction manifestations (ASLE and ISLE). It has been extensively demonstrated that in brain ischemia there is an increase in serum S100B protein levels (8, 23, 24), which may be correlated with the extent of brain damage (7) and could predict the early outcome in stroke patients (24). In our study, four patients in the NPSLE group had stroke at least 2 weeks before S100B measurement, suggesting that the observed increases in serum S100B levels in these patients were related more to SLE than to the stroke event itself (24). The other NPSLE patients, with transverse myelitis and chorea, also presented clearly higher levels of this protein in serum. Although there was only one case of myelitis in this study, taking into account our previous finding that S100B can be a marker of a chronic myelopathy related to the HTLV-1 retrovirus (21), the putative role of S100B as a marker of acute myelopathy emerges as a point to be investigated. Besides the evident neurologic lesions observed in these six patients, another factor that could contribute to increase the content of serum S100B is the astrocytic immunological response in the CNS, characterized by cytokine release. Accordingly, it has been shown that CSF S100B levels are increased in multiple sclerosis (11), which is an autoimmune disease with primary CNS involvement, and that interleukin-1 (IL-1) and IL-6 are increased in the CSF of NPSLE patients (20). Moreover, IL-1 in vivo is known to induce astrogliosis (19), which could be related to the increased expression of S100B reported here, leading to dystrophic neurites and calcium-mediated neuronal cell loss (10, 19).
In our study, we observed that serum S100B levels were increased not only in the NPSLE group but also in the ISLE and ASLE groups compared to controls, which was an unexpected result, since there was no evidence of neurologic involvement in these patients on clinical and laboratory evaluation. Based on previous literature findings, we offer two hypotheses to explain these results. First, it has been demonstrated that SLE patients without previous neuropsychiatric events frequently have cerebrovascular lesions as observed in magnetic resonance imaging (10, 14) and in single-photon-emission computed tomography (15). In addition, in another study, not only was the neurometabolism measured by proton magnetic resonance spectroscopy altered in patients with active or prior major NPSLE, but also some grade of alteration was present in all others SLE groups (18). Furthermore, some authors suggest that serum S100B levels could be a more sensitive marker than imaging methods in certain events of brain damage (6, 7). Whether subclinical involvement really account for our results, magnetic resonance imaging and single-photon-emission computed tomography studies could be useful to clarify this point. Second, it is known that corticosteroid therapy affects astrocyte parameters, such as glial fibrillary acidic protein and glutamine synthase expression (22). However, to our knowledge, steroid effects on S100B expression were reported only in vitro (13). Nevertheless, in our study, all patients from the NPSLE and ASLE groups were undergoing corticosteroid therapy, whereas in the ISLE group (which also showed increased S100B levels compared to controls) only 38.5% were receiving prednisone, so corticosteroid effects on astrocytes by themselves probably could not account for all of the results observed. In order to confirm the effects of corticosteroids on serum S100B levels, it will be necessary to perform studies with patients undergoing chronic corticosteroid treatment and with an evident absence of neurologic involvement, such as rheumatoid arthritis patients.
These preliminary findings need additional investigation in order to clarify whether the measurement of serum S100B protein levels could be a useful and complementary tool in the evaluation of CNS involvement in SLE patients. Since 19 neuropsychiatric syndromes in SLE have been recently defined by the ACR (1), further prospective trials, involving a larger number of patients with different forms of NPSLE and non-NPSLE manifestations, are ongoing in our groups in order to address many fundamental questions, such as predictive and prognostic value as well as correlation between disease activity and serum S100B levels.
Acknowledgments
This work was supported by CNPq and PRONEX no. 41960904.
REFERENCES
- 1.American College of Rheumatology. 1999. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 42:599–608. [DOI] [PubMed] [Google Scholar]
- 2.Donato, R. 1999. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim. Biophys. Acta 1450:191–231. [DOI] [PubMed] [Google Scholar]
- 3.Gazzolo, D., P. Vinesi, M. Bartocci, M. C. Geloso, W. Bonacci, G. Serra, K. G. Haglid, and F. Michetti. 1999. Elevated S100 blood level as an early indicator of intraventricular hemorrhage in preterm infants. Correlation with cerebral Doppler velocimetry. J. Neurol. Sci. 170:32–35. [DOI] [PubMed] [Google Scholar]
- 4.Gonçalves, D. S., G. Lenz, J. D. Karl, C. A. Gonçalves, and R. Rodnight. 2000. Extracellular S100B protein modulates ERK in astrocyte cultures. NeuroReport 11:807–809. [DOI] [PubMed] [Google Scholar]
- 5.Heizmann, C. W. 1999. Ca2+-binding S100 proteins in the central nervous system. Neurochem. Res. 24:1097–1100. [DOI] [PubMed] [Google Scholar]
- 6.Ingebrigtsen, T., B. Romner, P. Kongstad, and B. Langbakk. 1995. Increased serum concentration of protein S-100 after minor head injury: a biochemical serum marker with prognostic value? J. Neurol. Neurosurg. Psych. 59:103–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingebrigtsen, T., K. Waterloo, E. A. Jacobsen, B. Langbakk, and B. Romner. 1999. Traumatic brain damage in minor head injury: relation of serum S-100 protein measurements to magnetic resonance imaging and neurobehavioural outcome. Neurosurgery 45:468–475. [DOI] [PubMed] [Google Scholar]
- 8.Jonsson, H., P. Johnsson, C. Alling, S. Westaby, and S. Blomquist. 1998. Significance of serum S100 release after coronary artery bypass grafting. Ann. Thorac. Surg. 65:1639–1644. [DOI] [PubMed] [Google Scholar]
- 9.Kalunian, K. C. 1997. Definition, classification, activity and damage indices, p.19–29. In D. J. Wallace and B. H. Hahn (ed), Dubois’ lupus erythematosus. Williams & Wilkins, Baltimore, Md.
- 10.Kozora, E., S. G. West, B. L. Kotzin, L. Julian, S. Porter, and E. Bigler. 1998. Magnetic resonance imaging abnormalities and cognitive deficits in systemic lupus erythematous patients without overt central nervous system disease. Arthritis Rheum. 41:41–47. [DOI] [PubMed] [Google Scholar]
- 11.Lamers, K. J., B. G. van Engelen, F. J. Gabreels, O. R. Hommes, G. F. Borm, and R. A. Wevers. 1995. Cerebrospinal neuron-specific enolase, S-100 and myelin basic protein in neurological disorders. Acta Neurol. Scand. 92:247–251. [DOI] [PubMed] [Google Scholar]
- 12.Lara, D. R., C. S. Gama, P. Belmonte-de-Abreu, L. V. C. Portela, C. A. Gonçalves, M. Fonseca, S. Hauck, and D. O. Souza. 2001. Increased serum S100B protein in schizophrenia: a study in medication-free patients. J. Psychiat. Res. 35:11–14. [DOI] [PubMed] [Google Scholar]
- 13.Niu, H., D. A. Hinkle, and P. M. Wise. 1997. Dexamethasone regulates basic fibroblast growth factor, nerve growth factor and S100beta expression in cultured hippocampal astrocytes. Mol. Brain Res. 51:97–105. [DOI] [PubMed] [Google Scholar]
- 14.Nomura, K., S. Yamano, Y. Ikeda, H. Yamada, T. Fujimoto, S. Minami, R. Fukui, M. Takaoka, Y. Yamamoto, and K. Dohi. 1999. Asymptomatic cerebrovascular lesions detected by magnetic resonance imaging in patients with systemic lupus erythematosus lacking a history of neuropsychiatric events. Intern. Med. 38:785–795. [DOI] [PubMed] [Google Scholar]
- 15.Sabbadini, M. G., A. A. Manfredi, E. Bozzolo, L. Ferrario, C. Rugarli, R. Scorza, L. Origgi, M. Vanoli, O. Gambini, L. Vanzulli, D. Croce, A. Campana, C. Messa, F. Fazio, A. Tincani, G. Anzola, R. Cattaneo, A. Padovani, R. Gasparotti, R. Gerli, R. Quartesan, M. Piccirilli, A. Farsi, E. Emmi, and A. Passaleva. 1999. Central nervous system involvement in systemic lupus erythematous patients without overt neuropsychiatric manifestations. Lupus 8:11–19. [DOI] [PubMed] [Google Scholar]
- 16.Sanna, G., M. Piga, J. W. Terryberry, M. T. Peltz, S. Giagheddu, L. Satta, A. Ahmed, A. Cauli, C. Montaldo, G. Passiu, J. B. Peter, Y. Shoenfeld, and A. Mathieu. 2000. Central nervous system involvement in systemic lupus erythematosus: cerebral imaging and serological profile in patients with and without overt neuropsychiatric manifestations. Lupus 9:573–583. [DOI] [PubMed] [Google Scholar]
- 17.Sheng, J. G., R. E. Mrak, C. R. Rovnaghi, E. Kozlowska, L. J. Van Eldik, and W. S. Griffin. 1996. Human brain S100B and S100B mRNA expression increases with age: pathogenic implications for Alzheimer’s disease. Neurobiol. Aging 17:359–363. [DOI] [PubMed] [Google Scholar]
- 18.Sibbittt, W. L., Jr., L. J. Haseler, R. R. Griffey, S. D. Friedman, and W. M. Brooks. 1997. Neurometabolism of active neuropsychiatric lupus determined by proton MR spectroscopy. Am. J. Neuroradiol. 18:1271–1277. [PMC free article] [PubMed] [Google Scholar]
- 19.Stanley, L. C., R. E. Mrak, R. C. Woody, L. J. Perrot, S. Zhang, D. R. Marshak, S. J. Nelson, and W. S. Griffin. 1994. Glial cytokines as neuropathogenic factors in HIV infection: pathogenic similarities to Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 53:231–238. [DOI] [PubMed] [Google Scholar]
- 20.Varela, J. A., A. D. Hoey, and D. A. Segovia. 1992. Interleukin-1 and interleukin-6 activities are increased in cerebrospinal fluid of patients with CNS lupus erythematosus and correlate with local late T-cell activation markers. Lupus 1:111–117. [DOI] [PubMed] [Google Scholar]
- 21.Walz, R., L. V. Portela, A. B. Tort, E. C. Neto, L. N. Fernandes, C. A. Goncalves, and D. O. Souza. 2000. Serum S100B levels in patients with HTLV-I associated myelopathy/tropical spastic paraparesis. Neurology 54:2021–2022. [DOI] [PubMed] [Google Scholar]
- 22.Weir, M. D., and D. G. Thomas. 1984. Effect of dexamethasone on glutamine synthetase and glial fibrillary acidic protein in normal and transformed astrocytes. Clin. Neuropharmacol. 7:303–306. [DOI] [PubMed] [Google Scholar]
- 23.Wong, C. H., S. J. Rooney, and R. S. Bonser. 1999. S100beta release in hypothermic circulatory arrest and coronary artery surgery. Ann. Thoracic Surg. 67:1911–1914. [DOI] [PubMed] [Google Scholar]
- 24.Wunderlich, M. T., A. D. Ebert, T. Kratz, M. Goertler, S. Jost, and M. Herrmann. 1999. Early neurobehavioural outcome after stroke is related to release of neurobiochemical markers of brain damage. Stroke 30:1190–1195. [DOI] [PubMed] [Google Scholar]