Abstract
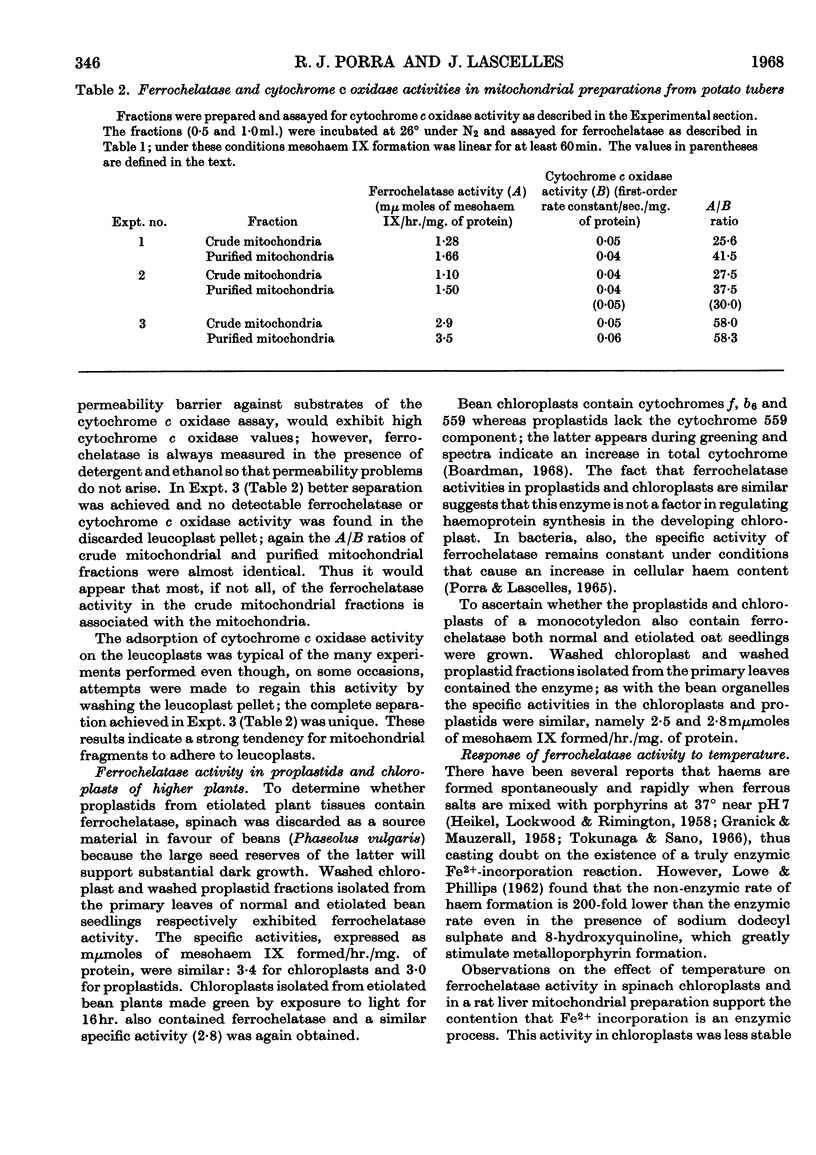
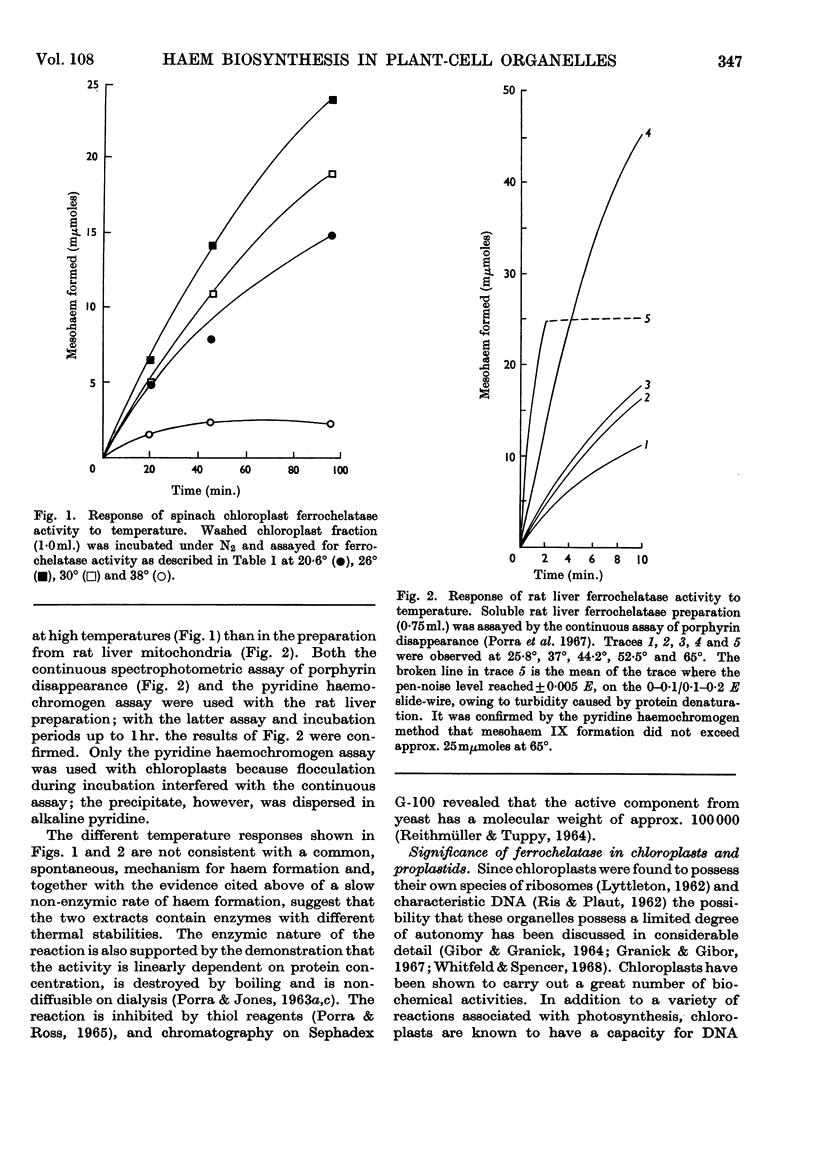
1. Ferrochelatase was demonstrated in the chloroplasts and proplastids isolated from the primary leaves of beans (a dicotyledon) and oats (a monocotyledon). It was also detected in chloroplasts from etiolated bean seedlings made green by illumination before being harvested. The specific activities of the three types of bean organelles are similar, as are the specific activities of the oat proplastids and chloroplasts. 2. Chloroplasts from young spinach leaves also contain ferrochelatase; these chloroplasts were tested for their ability to form magnesium tetrapyrroles and found unable to catalyse the insertion of Mg2+ into mesoporphyrin IX. 3. Ferrochelatase was also detected in potato tuber mitochondria. 4. Ferrochelatase activity in these plant preparations is much less stable on storage than similar preparations from bacteria and animal tissues. 5. Temperature affects the activities of spinach chloroplast ferrochelatase and rat liver ferrochelatase differently. Activity of the chloroplast enzyme increases as the temperature rises from 20·6° to 26°, but becomes increasingly inactivated as the temperature rises further to 38°. The initial velocity of the mammalian enzyme, however, increases as the temperature rises from 25·8° to 65°, but the enzyme is inactivated after several minutes at 65°.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER E. W., RUCCIA M., CORWIN A. H. THE PREPARATION OF MESOPORPHYRIN IX AND ETIOPORPHYRIN 3. Anal Biochem. 1964 Aug;8:512–518. doi: 10.1016/0003-2697(64)90249-0. [DOI] [PubMed] [Google Scholar]
- Brooks J. L., Stumpf P. K. Fat metabolism in higher plants. XXXIX. Properties of a soluble fatty acid synthesizing system from lettuce chloroplasts. Arch Biochem Biophys. 1966 Sep 26;116(1):108–116. doi: 10.1016/0003-9861(66)90019-1. [DOI] [PubMed] [Google Scholar]
- CLARK P., WALSH R. J. Synthesis of haem by circulating blood cells. Nature. 1959 Nov 28;184(Suppl 22):1730–1731. doi: 10.1038/1841730b0. [DOI] [PubMed] [Google Scholar]
- GIBOR A., GRANICK S. PLASTIDS AND MITOCHONDRIA: INHERITABLE SYSTEMS. Science. 1964 Aug 14;145(3633):890–897. doi: 10.1126/science.145.3635.890. [DOI] [PubMed] [Google Scholar]
- GRANICK S., MAUZERALL D. Pbrphyrin biosynthesis in erythrocytes. II. Enzymes converting gamma-aminolevulinic acid to coproporphyrinogen. J Biol Chem. 1958 Jun;232(2):1119–1140. [PubMed] [Google Scholar]
- Granick S., Gibor A. The DNA of chloroplasts, mitochondria and centrioles. Prog Nucleic Acid Res Mol Biol. 1967;6:143–186. doi: 10.1016/s0079-6603(08)60526-7. [DOI] [PubMed] [Google Scholar]
- HEIKEL T., LOCKWOOD W. H., RIMINGTON C. Formation of non-enzymic haem. Nature. 1958 Aug 2;182(4631):313–313. doi: 10.1038/182313a0. [DOI] [PubMed] [Google Scholar]
- KLEIN J. R., KRUEGER R. C., MELNICK I. Formation of heme by broken-cell preparations of duck erythrocytes. Arch Biochem Biophys. 1956 Oct;64(2):302–310. doi: 10.1016/0003-9861(56)90273-9. [DOI] [PubMed] [Google Scholar]
- LABBE R. F., HUBBARD N., CAUGHEY W. S. Porphyrin specificity of ferro:protoporphyrin chelatase from rat liver. Biochemistry. 1963 Mar-Apr;2:372–374. doi: 10.1021/bi00902a033. [DOI] [PubMed] [Google Scholar]
- LABBE R. F., NISHIDA G. A new method of hemin isolation. Biochim Biophys Acta. 1957 Nov;26(2):437–437. doi: 10.1016/0006-3002(57)90033-1. [DOI] [PubMed] [Google Scholar]
- LOCHHEAD A. C., GOLDBERG A. The enzymic formation of haem by human and rat tissues. Biochem J. 1961 Jan;78:146–150. doi: 10.1042/bj0780146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWE M. B., PHILLIPS J. N. A possible mode of action of some anti-fungal and anti-bacterial chelating agents. Nature. 1962 Jun 16;194:1058–1059. doi: 10.1038/1941058a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LYTTLETON J. W. Isolation of ribosomes from spinach chloroplasts. Exp Cell Res. 1962 Mar;26:312–317. doi: 10.1016/0014-4827(62)90183-0. [DOI] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 1. Assay and properties of ferrochelatase from a pig-liver mitochondrial extract. Biochem J. 1963 Apr;87:181–185. doi: 10.1042/bj0870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 2. An in vestigation of the role offerrochelatase in the biosynthesis of various haem prosthetic groups. Biochem J. 1963 Apr;87:186–192. doi: 10.1042/bj0870186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., LASCELLES J. HAEMOPROTEINS AND HAEM SYNTHESIS IN FACULTATIVE PHOTOSYNTHETIC AND DENITRIFYING BACTERIA. Biochem J. 1965 Jan;94:120–126. doi: 10.1042/bj0940120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., ROSS B. D. HAEM SYNTHASE AND COBALT PORPHYRIN SYNTHASE IN VARIOUS MICRO-ORGANISMS. Biochem J. 1965 Mar;94:557–562. doi: 10.1042/bj0940557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra R. J., Vitols K. S., Labbe R. F., Newton N. A. Studies on ferrochelatase. The effects of thiols and other factors on the determination of activity. Biochem J. 1967 Aug;104(2):321–327. doi: 10.1042/bj1040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIETHMUELLER G., TUPPY H. HAEMSYNTHETASE (FERROCHELATASE) IN SACCHAROMYCES CEREVISIAE NACH AEROBEM UND ANAEROBEM WACHSTUM. Biochem Z. 1964 Sep 28;340:413–420. [PubMed] [Google Scholar]
- RIMINGTON C., TOOTH B. E. Role of mitochondria in the in vitro formation of protoporphyrin and haem. J Biochem. 1961 Jun;49:456–467. doi: 10.1093/oxfordjournals.jbchem.a127328. [DOI] [PubMed] [Google Scholar]
- RIS H., PLAUT W. Ultrastructure of DNA-containing areas in the chloroplast of Chlamydomonas. J Cell Biol. 1962 Jun;13:383–391. doi: 10.1083/jcb.13.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEMAL J., SPENCER D., KIM Y. T., WILDMAN S. G. PROPERTIES OF A RIBONUCLEIC ACID SYNTHESIZING SYSTEM IN CELL-FREE EXTRACTS OF TOBACCO LEAVES. Biochim Biophys Acta. 1964 Oct 16;91:205–216. doi: 10.1016/0926-6550(64)90243-9. [DOI] [PubMed] [Google Scholar]
- SPENCER D., WILDMAN S. G. THE INCORPORATION OF AMINO ACIDS INTO PROTEIN BY CELL-FREE EXTRACTS FROM TOBACCO LEAVES. Biochemistry. 1964 Jul;3:954–959. doi: 10.1021/bi00895a019. [DOI] [PubMed] [Google Scholar]
- Smillie R. M., Graham D., Dwyer M. R., Grieve A., Tobin N. F. Evidence for the synthesis in vivo of proteins of the Calvin cycle and of the photosynthetic electron-transfer pathway on chloroplast ribosomes. Biochem Biophys Res Commun. 1967 Aug 23;28(4):604–610. doi: 10.1016/0006-291x(67)90356-7. [DOI] [PubMed] [Google Scholar]
- Spencer D. Protein synthesis by isolated spinach chloroplasts. Arch Biochem Biophys. 1965 Aug;111(2):381–390. doi: 10.1016/0003-9861(65)90200-6. [DOI] [PubMed] [Google Scholar]
- Spencer D., Whitfeld P. R. DNA synthesis in isolated chloroplasts. Biochem Biophys Res Commun. 1967 Aug 23;28(4):538–542. doi: 10.1016/0006-291x(67)90347-6. [DOI] [PubMed] [Google Scholar]
- Spencer D., Whitfeld P. R. Ribonucleic acid synthesizing activity of spinach chloroplasts and nuclei. Arch Biochem Biophys. 1967 Aug;121(2):336–345. doi: 10.1016/0003-9861(67)90085-9. [DOI] [PubMed] [Google Scholar]
- TSUI C. The role of zinc auxin synthesis in the tomato plant. Am J Bot. 1948 Mar;35(3):172–179. [PubMed] [Google Scholar]


