Abstract
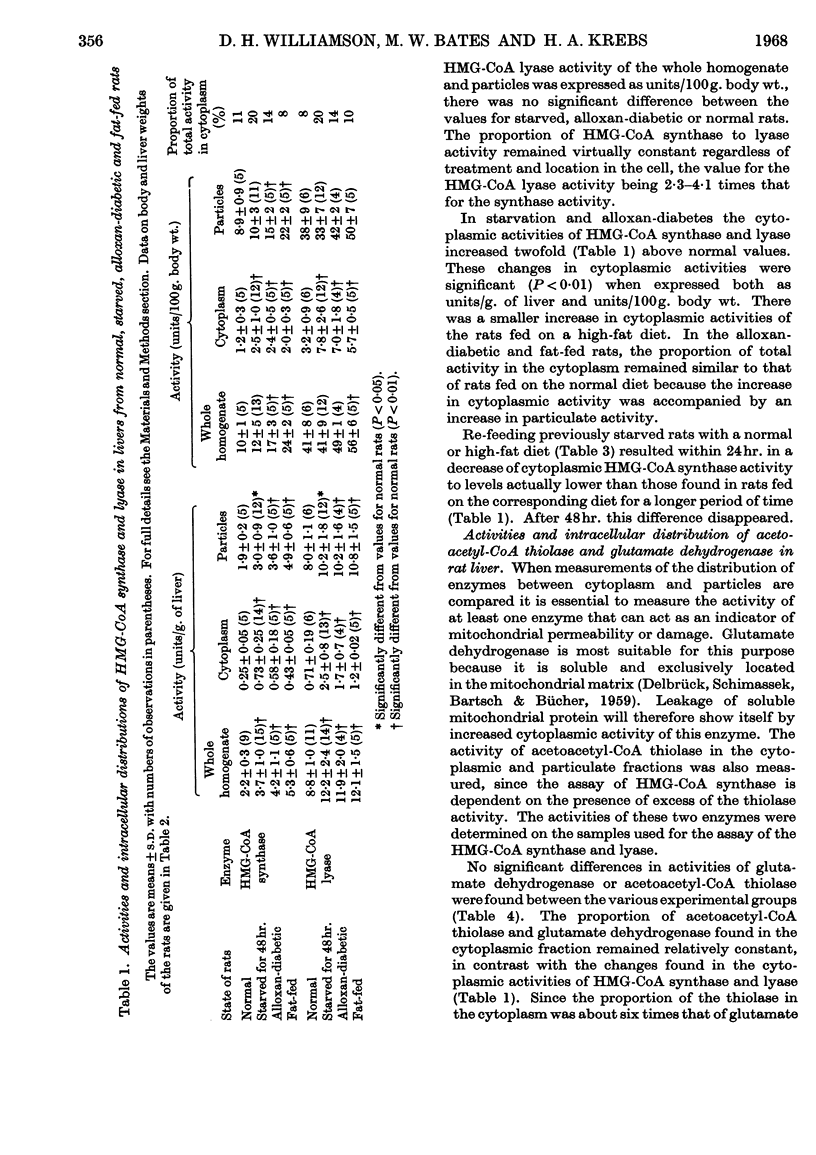
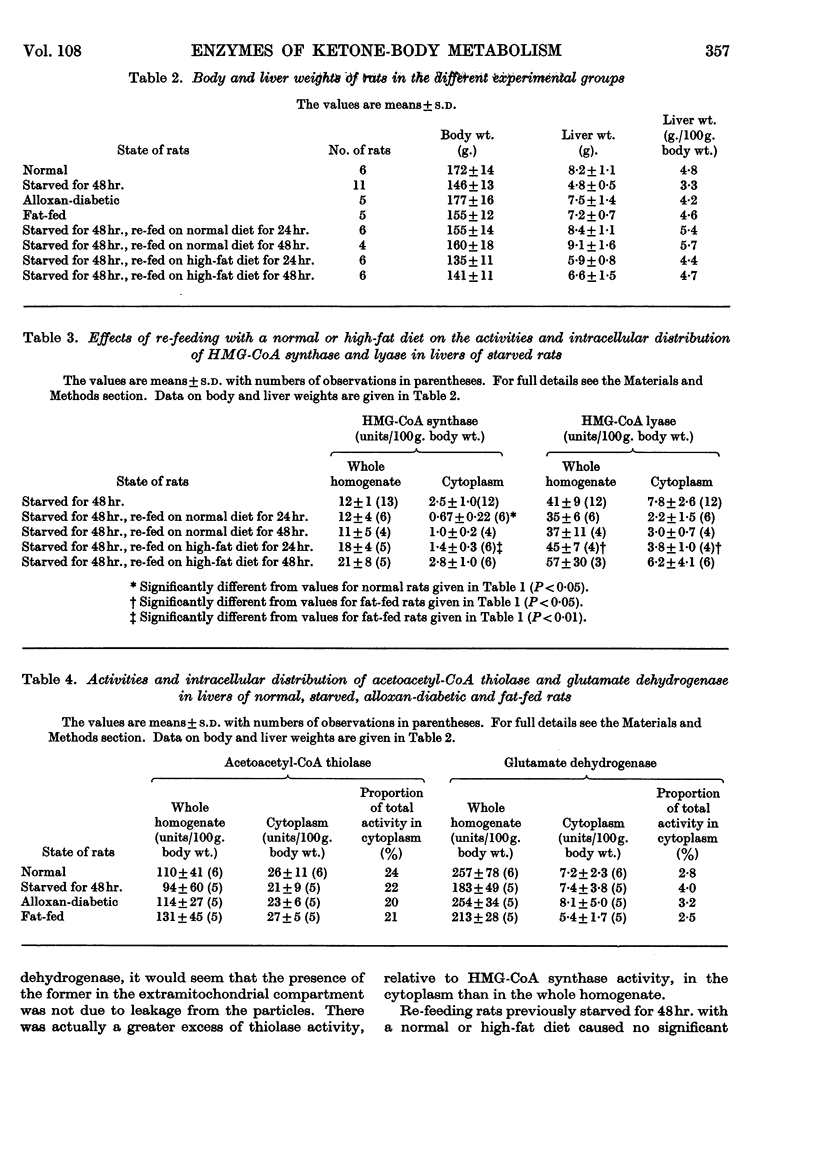
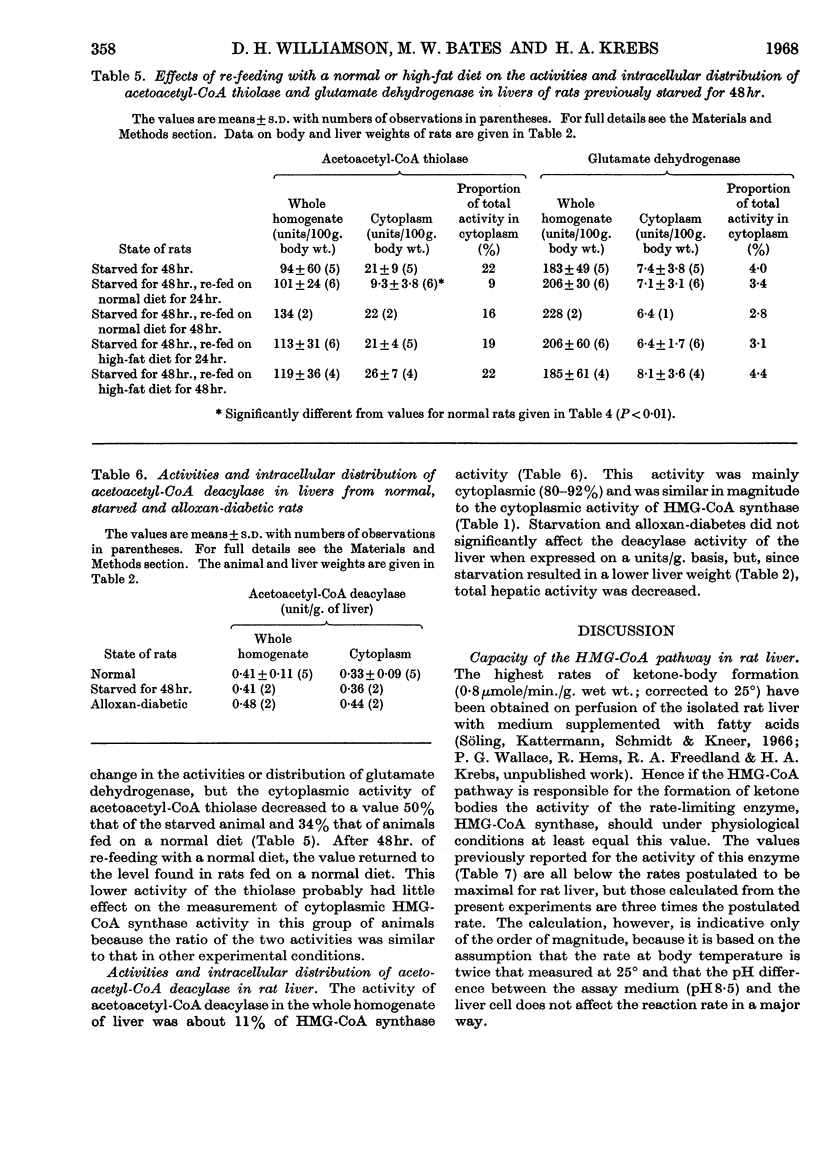
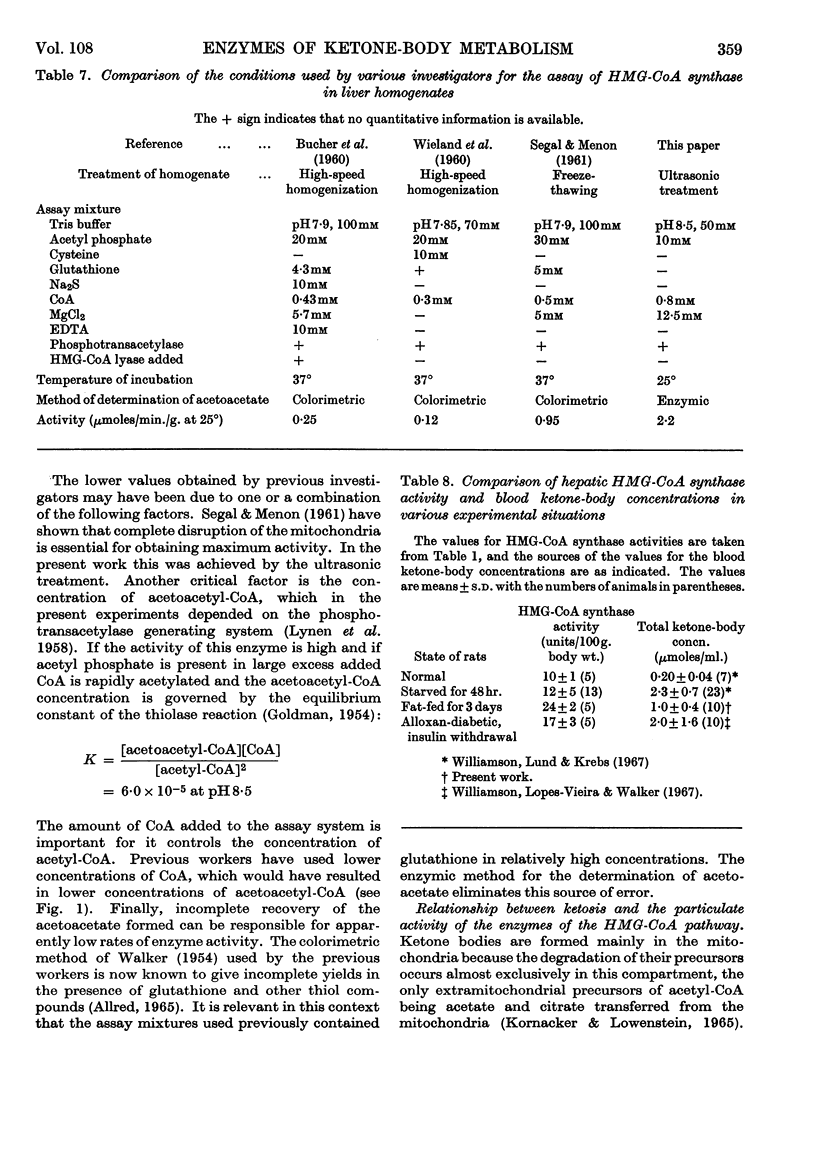
1. The activities of hydroxymethylglutaryl-CoA synthase and lyase in rat liver were found to be two- to 15-fold greater than those reported by other authors under similar conditions. 2. When expressed on the basis of body weight, no appreciable differences were found between the activities of hydroxymethylglutaryl-CoA synthase in whole homogenates of livers from normal and starved rats. The synthase activity increased by 70% and 140% in livers of alloxan-diabetic rats and rats fed on a high-fat diet respectively. 3. Hydroxymethylglutaryl-CoA lyase activity showed no significant increases in starvation or alloxan-diabetes, but a 40% increase was found in fat-fed rats. 4. Less than 12% of the activities of both enzymes were found in the cytoplasmic fraction of normal liver. The cytoplasmic activity doubled in alloxan-diabetes and starvation; on feeding with a high-fat diet the increase, though significant, was less marked. 6. The intracellular distribution of glutamate dehydrogenase indicated that the changes in the cytoplasmic activities observed were not due to leakage from the mitochondria. 7. Feeding with a normal or high-fat diet after 48hr. starvation caused within 24hr. a decrease in the cytoplasmic activity of hydroxymethylglutaryl-CoA synthase to values lower than those found in rats fed on a corresponding diet for a longer period of time. 8. Acetoacetyl-CoA deacylase activity in liver was about 20% of that of hydroxymethylglutaryl-CoA synthase and was primarily located in the cytoplasm. Starvation or alloxan-diabetes did not alter the acetoacetyl-CoA deacylase activity. 9. It is concluded that variations in the concentrations of enzymes involved in acetoacetate synthesis play no major role in the regulation of ketone-body formation in starvation and alloxan-diabetes. The changes in the cytoplasmic activities of hydroxymethylglutaryl-CoA synthase and lyase suggest that acetoacetate synthesis can occur in the cytoplasm. This may play a role in the disposal of surplus acetyl-CoA arising in the cytoplasm when lipogenesis is inhibited.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLRED J. B. INTERFERENCE BY THIOLS IN ACETOACETATE DETERMINATION. Anal Biochem. 1965 Apr;11:138–143. doi: 10.1016/0003-2697(65)90053-9. [DOI] [PubMed] [Google Scholar]
- BUCHER N. L., OVERATH P., LYNEN F. beta-Hydroxy-beta-methyl-glutaryl coenzyme A reductase, cleavage and condensing enzymes in relation to cholesterol formation in rat liver. Biochim Biophys Acta. 1960 Jun 3;40:491–501. doi: 10.1016/0006-3002(60)91390-1. [DOI] [PubMed] [Google Scholar]
- Bergmeyer H. U., Gawehn K., Klotzsch H., Krebs H. A., Williamson D. H. Purification and properties of crystalline 3-hydroxybutyrate dehydrogenase from Rhodopseudomonas spheroides. Biochem J. 1967 Feb;102(2):423–431. doi: 10.1042/bj1020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDRICKSON D. S., GORDON R. S., Jr Transport of fatty acids. Physiol Rev. 1958 Oct;38(4):585–630. doi: 10.1152/physrev.1958.38.4.585. [DOI] [PubMed] [Google Scholar]
- GOLDMAN D. S. Studies on the fatty acid oxidizing system of animal tissues. VII. The beta-ketoacyl coenzyme A cleavage enzyme. J Biol Chem. 1954 May;208(1):345–357. [PubMed] [Google Scholar]
- HILZ H., KNAPPE J., RINGELMANN E., LYNEN F. Methylglutaconase, eine neue Hydratase, die am Stoffwechsel verzweigter Carbonsäuren beteiligt ist. Biochem Z. 1958;329(6):476–489. [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. M. CITRATE AND THE CONVERSION OF CARBOHYDRATE INTO FAT. THE ACTIVITIES OF CITRATE-CLEAVAGE ENZYME AND ACETATE THIOKINASE IN LIVERS OF STARVED AND RE-FED RATS. Biochem J. 1965 Jan;94:209–215. doi: 10.1042/bj0940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYNEN F., HENNING U., BUBLITZ C., SORBO B., KROPLIN-RUEFF L. Der chemische Mechanismus der Acetessigsäurebildung in der Leber. Biochem Z. 1958;330(4):269–295. [PubMed] [Google Scholar]
- STERN J. R., MILLER G. E. On the enzymic mechanism of acetoacetate synthesis. Biochim Biophys Acta. 1959 Oct;35:576–577. doi: 10.1016/0006-3002(59)90424-x. [DOI] [PubMed] [Google Scholar]
- STERN J. R. Optical properties of aceto-acetyl-S-coenzyme A and its metal chelates. J Biol Chem. 1956 Jul;221(1):33–44. [PubMed] [Google Scholar]
- Sauer F., Erfle J. D. On the mechanism of acetoacetate synthesis by guinea pig liver fractions. J Biol Chem. 1966 Jan 10;241(1):30–37. [PubMed] [Google Scholar]
- Tubbs P. K., Garland P. B. Variations in tissue contents of coenzyme A thio esters and possible metabolic implications. Biochem J. 1964 Dec;93(3):550–557. doi: 10.1042/bj0930550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER P. G. A colorimetric method for the estimation of acetoacetate. Biochem J. 1954 Dec;58(4):699–704. doi: 10.1042/bj0580699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIELAND O., WEISS L. Increase in liver acetyl-coenzyme A during ketosis. Biochem Biophys Res Commun. 1963 Feb 18;10:333–339. doi: 10.1016/0006-291x(63)90534-5. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lopes-Vieira O., Walker B. Concentrations of free glucogenic amino acids in livers of rats subjected to various metabolic stresses. Biochem J. 1967 Aug;104(2):497–502. doi: 10.1042/bj1040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]


