Abstract
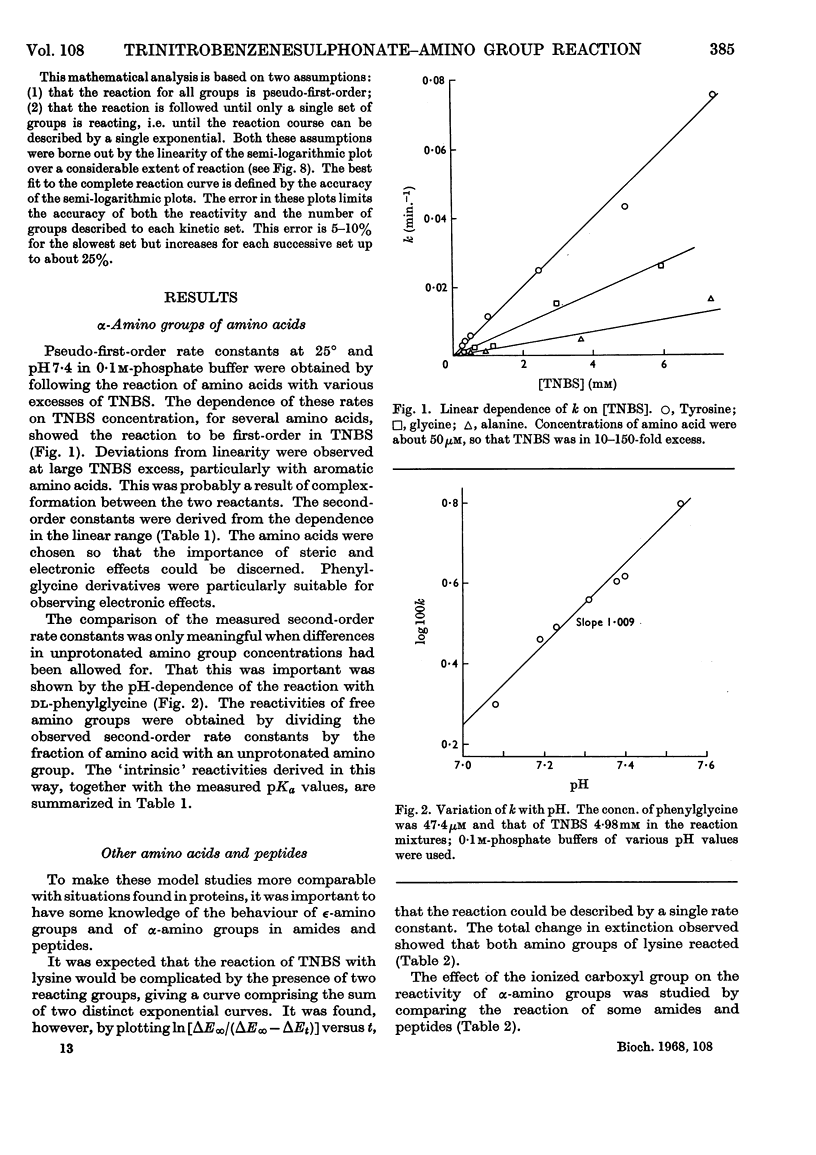
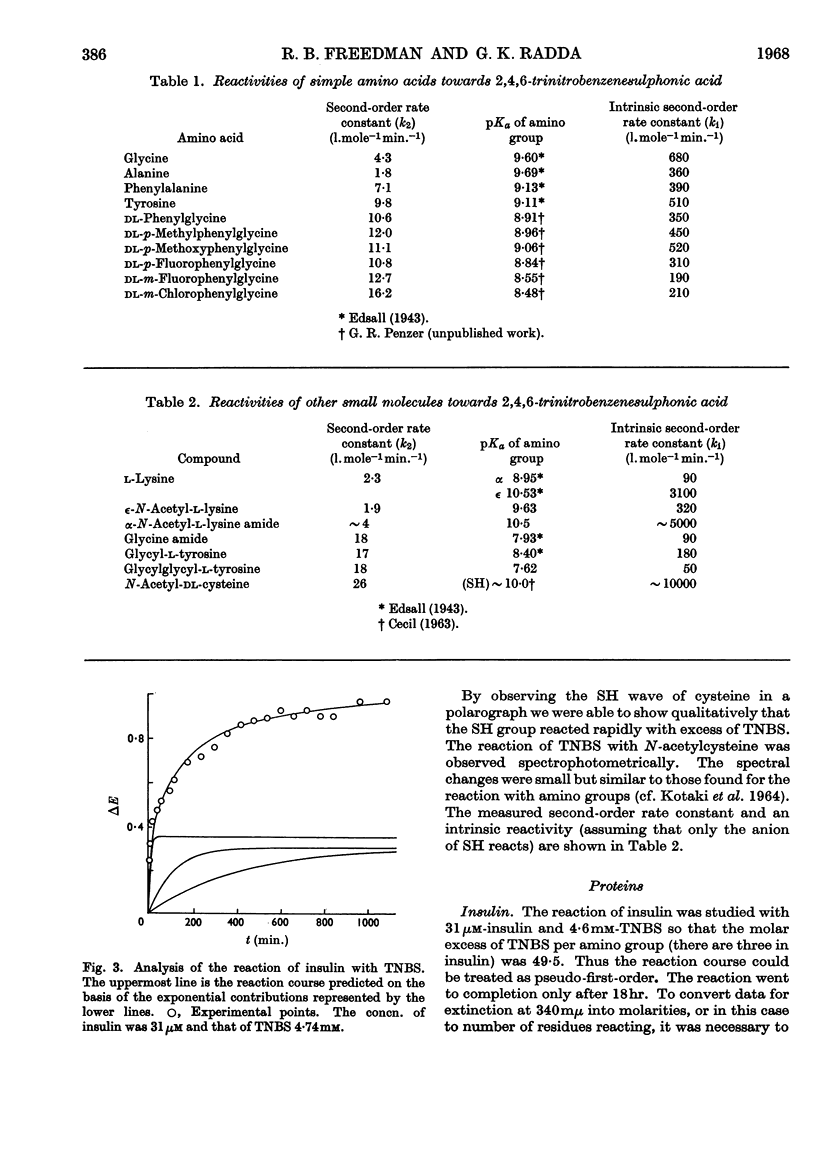
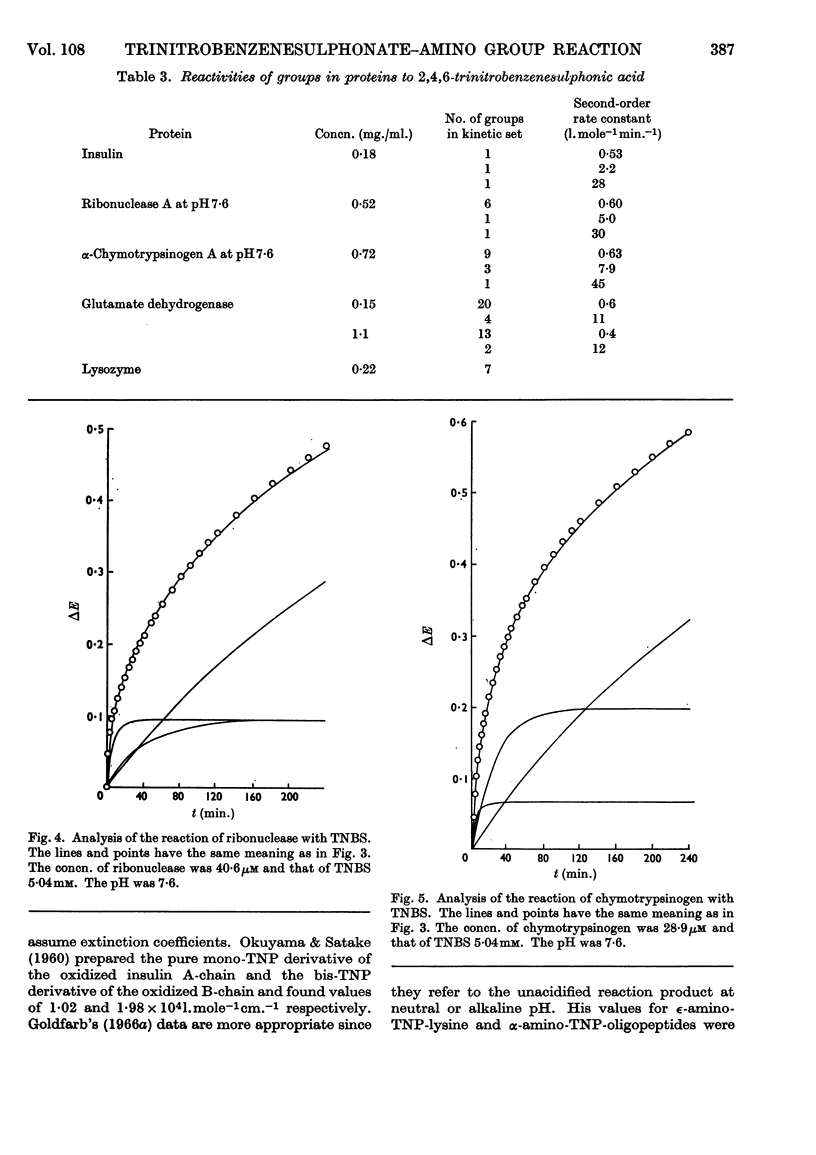
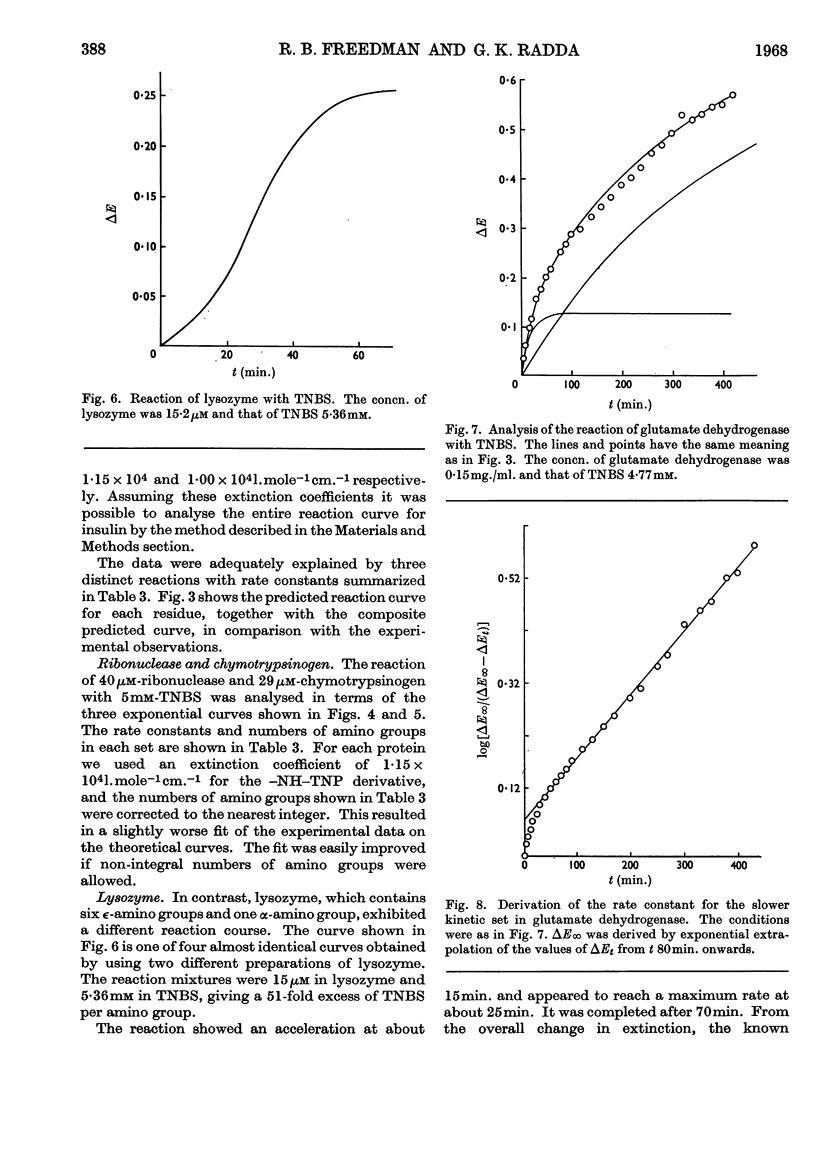
1. The kinetics of the reaction of 2,4,6-trinitrobenzenesulphonic acid with various amino acids, peptides and proteins were studied by spectrophotometry. 2. The reaction of the α- and ∈-amino groups in simple amino acids was found to be second-order, and the unprotonated amino group was shown to be the reactive species. 3. By allowing for the concentration of unreactive −NH3+ group, intrinsic reactivities for the free amino groups were derived and shown to be correlated with the basicities. 4. The SH group of N-acetylcysteine was found to be more reactive to 2,4,6-trinitrobenzenesulphonic acid than most amino groups. 5. The reactions of insulin, chymotrypsinogen and ribonuclease with 2,4,6-trinitrobenzenesulphonic acid were analysed in terms of three exponential rate curves, each referring to one or more amino groups of the proteins. 6. The reaction of lysozyme with 2,4,6-trinitrobenzenesulphonic acid was found to display an acceleration effect. 7. From the reaction of 2,4,6-trinitrobenzenesulphonic acid with glutamate dehydrogenase at several enzyme concentrations, it was possible to discern two sets of amino groups of different reactivity, and to show that the number of groups in each set was decreased by aggregation of the enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley P. M., Radda G. K. Conformational changes and the regulation of glutamate-dehydrogenase activity. Biochem J. 1966 Jan;98(1):105–111. doi: 10.1042/bj0980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHERVENKA C. H., WILCOX P. E. Chemical derivatives of chymotrypsinogen. I. Reaction with carbon disulfide. J Biol Chem. 1956 Oct;222(2):621–634. [PubMed] [Google Scholar]
- Colman R. F., Frieden C. On the role of amino groups in the structure and function of glutamate dehydrogenase. I. Effect of acetylation on catalytic and regulatory properties. J Biol Chem. 1966 Aug 25;241(16):3652–3660. [PubMed] [Google Scholar]
- Colman R. F., Frieden C. On the role of amino groups in the structure and function of glutamate dehydrogenase. II. Effect of acetylation on molecular properties. J Biol Chem. 1966 Aug 25;241(16):3661–3670. [PubMed] [Google Scholar]
- GREENLEE L., HANDLER P. XANTHINE OXIDASE. VII. INHIBITION BY AMINO GROUP REAGENTS. J Biol Chem. 1964 Apr;239:1096–1101. [PubMed] [Google Scholar]
- Goldfarb A. R. A kinetic study of the reactions of amino acids and peptides with trinitrobenzenesulfonic acid. Biochemistry. 1966 Aug;5(8):2570–2574. doi: 10.1021/bi00872a013. [DOI] [PubMed] [Google Scholar]
- Goldfarb A. R. Heterogeneity of amino groups in proteins. I. Human serum albumin. Biochemistry. 1966 Aug;5(8):2574–2578. doi: 10.1021/bi00872a014. [DOI] [PubMed] [Google Scholar]
- HELLERMAN L., SCHELLENBERG K. A., REISS O. K. L-glutamic acid dehydrogenase. II. Role of enzyme sulfhydryl groups. J Biol Chem. 1958 Dec;233(6):1468–1478. [PubMed] [Google Scholar]
- HIRS C. H. Dinitrophenyiribonucleases. Brookhaven Symp Biol. 1962 Dec;15:154–183. [PubMed] [Google Scholar]
- Haynes R., Osuga D. T., Feeney R. E. Modification of amino groups in inhibitors of proteolytic enzymes. Biochemistry. 1967 Feb;6(2):541–547. doi: 10.1021/bi00854a023. [DOI] [PubMed] [Google Scholar]
- KOTAKI A., HARADA M., YAGI K. REACTION BETWEEN SULFHYDRYL COMPOUNDS AND 2,4,6-TRINITROBENZENE-1-SULFONIC ACID. J Biochem. 1964 May;55:553–561. [PubMed] [Google Scholar]
- LI C. H. Preparation and properties of dinitrophenyl-NH(epsilon)-insulin. Nature. 1956 Dec 22;178(4547):1402–1402. doi: 10.1038/182001a0. [DOI] [PubMed] [Google Scholar]
- Matthews B. W., Sigler P. B., Henderson R., Blow D. M. Three-dimensional structure of tosyl-alpha-chymotrypsin. Nature. 1967 May 13;214(5089):652–656. doi: 10.1038/214652a0. [DOI] [PubMed] [Google Scholar]
- OLSON J. A., ANFINSEN C. B. The crystallization and characterization of L-glutamic acid dehydrogenase. J Biol Chem. 1952 May;197(1):67–79. [PubMed] [Google Scholar]
- RAY W. J., Jr, KOSHLAND D. E., Jr A method for characterizing the type and numbers of groups involved in enzyme action. J Biol Chem. 1961 Jul;236:1973–1979. [PubMed] [Google Scholar]
- SHINODA T. THE APPARENT HIGH REACTIVITY OF SOME AMINO GROUPS OF NATIVE HEMOGLOBIN. Biochim Biophys Acta. 1965 Feb 15;97:382–384. doi: 10.1016/0304-4165(65)90117-0. [DOI] [PubMed] [Google Scholar]
- SRI RAM J., BIER M., MAURER P. H. Chemical modifications of proteins and their significance in enzymology, immunochemistry, and related subjects. Adv Enzymol Relat Subj Biochem. 1962;24:105–160. doi: 10.1002/9780470124888.ch3. [DOI] [PubMed] [Google Scholar]
- SUND H., AKESON A. DIE AMINOSAEUREZUSAMMENSETZUNG DER GLUTAMINSAEUREDEHYDROGENASE AUS RINDERLEBER. Biochem Z. 1964 Sep 28;340:421–435. [PubMed] [Google Scholar]
- TSOU C. L. Relation between modification of functional groups of proteins and their biological activity. I.A graphical method for the determination of the number and type of essential groups. Sci Sin. 1962 Nov;11:1535–1558. [PubMed] [Google Scholar]
- Wyckoff H. W., Hardman K. D., Allewell N. M., Inagami T., Johnson L. N., Richards F. M. The structure of ribonuclease-S at 3.5 A resolution. J Biol Chem. 1967 Sep 10;242(17):3984–3988. [PubMed] [Google Scholar]


