Abstract
The variable regions of the heavy and light chains of the protective murine monoclonal antibody (MAb) 2H1 (m2H1) were expressed with the human constant region genes for immunoglobulin G2 (IgG2) and kappa, respectively, to construct a chimeric antibody (ch2H1). ch2H1 retains the specificity of the parent MAb, exhibits biological activity, and lacks the toxicity of the parent murine IgG1 in chronically infected mice.
Cryptococcus neoformans is a fungus that causes life-threatening meningoencephalitis in 6 to 8% of patients with advanced human immunodeficiency virus (10). Cryptococcal meningoencephalitis in patients with AIDS is often incurable, because antifungal therapy does not eradicate the infection in the setting of impaired immunity (17). The difficulties associated with treatment of cryptococcal infections have stimulated interest in the potential usefulness of immunotherapy, including antibody therapy (1). A murine monoclonal antibody (MAb) to the C. neoformans capsular glucuronoxylomannan (GXM) (3) is currently in phase I evaluation as adjunctive therapy for cryptococcal meningitis. However, since murine MAbs can elicit human anti-mouse antibody responses that reduce their efficacy in therapy (2), it is desirable to develop antibodies with a human constant region to reduce antigenicity and provide better human effector functions.
Currently, little information is available regarding the relative efficacy of human constant regions against fungi. We are aware of only two reports in the literature evaluating human antibody function against fungi. Zebedee et al. generated two immunoglobulin G1 (IgG1) mouse-human chimeric antibodies from murine MAbs to C. neoformans GXM (21). One chimeric antibody, generated from the murine IgM MAb 2D10, lost binding affinity when converted to IgG1 and was not studied further (21). The second mouse-human IgG1, generated from the murine IgG1 MAb 18B7, promoted phagocytosis and prolonged survival in mice (21). Another group generated two human IgM MAbs by Epstein-Barr virus transformation of peripheral B cells; one has been shown to be opsonic and protective in the presence of complement (11, 22).
Studies of murine isotypes have shown major differences in IgG subclass efficacy against C. neoformans in vivo and in vitro (14, 19, 20). At this time, there is not sufficient information available to predict which human isotypes may be most effective against cryptococcal infection. Here, we report the construction and characterization of a mouse-human IgG2 antibody derived from the protective murine MAb 2H1, which has been the subject of extensive studies (reviewed in reference 18). Although there is no exact correspondence with regard to function, murine IgG3 and human IgG2 are usually made in response to polysaccharide antigens like the cryptococcal GXM.
Chimeric 2H1 (ch2H1) was constructed by amplifying MAb 2H1 variable regions of the heavy and light chains (VH and VL, respectively) and cloning the MAb into vector expressing the human IgG2 and kappa chains, respectively. Briefly, cDNA was made with oligonucleotide dT [5′ GCC GGA ATT CTA GAA GC(T) 3′] for amplification of the light chain. An IgG1 CH1-specific primer [5′ AGG TCT AGA A(CT)C TCC ACA CAC AGG (AG)(AG)C CAG TGG ATA GAC 3′] was used for amplification of the heavy chain. J primer and leader primer [5′ AGC GTC GAC TTA CGT TT(TG) ATT TCC A(GA)C TT(GT) GTC CC 3′ and 5′ GGGG ATA TCC ACC ATG AAG TTG CCT GTT AGG CTG TTG 3′, respectively] were used to amplify VL. J primer and leader primer (5′ CTT GGT GCT AGC TGA GGA GAC TGT GAG AGT G 3′ and 5′ GGG GAT ATC CACC ATG (AG)AC TTC GGG (TC)TG AGC T(TG)G GTT TT 3′, respectively) were used to amplify VH. The products were cloned into Bluescript KSII and digested with EcoRV, a poly (A) tail was added with TdT, and the products were sequenced. The VH clone was found to be missing the EcoRV site required for cloning into the expression vector, so the PCR was repeated with the same primers and with the VH clone with the missing site as a template. The clone was sequenced again. The SalI-EcoRV fragment containing the light chain V region was cloned into the kappa PCR expression vector. For the heavy chain V region, the NheI-EcoRV fragment containing the heavy chain V region was cloned, first into the IgG3 PCR expression vector and then subsequently moved into an IgG2 PCR expression vector.
A light chain producer was first made by transfecting 10 μg of the light chain vector containing the 2H1 VL linearized with PvuI into NSO/1 cells. The cells were selected on 1/2× HXM (3 μg of mycophenolic acid per ml). Surviving colonies were assayed by enzyme-linked immunosorbent assay (ELISA) and biosynthetically labeled, and 10 were subcloned. The highest-producing subclone was then transfected with 10 μg of the heavy chain vector linearized with PvuI. These cells were initially selected on 13 mM histidinol during the first feeding with drug selection. They were then fed with 10 mM histidinol on the second feeding. Surviving clones were assayed by ELISA for IgG2 production. Those scoring positive were biosynthetically labeled by growth in [35S]methionine. The secreted protein was precipitated with anti-human IgG, and then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Selected clones synthesizing the correct product were subcloned, and the highest-producing subclone was designated for further study. ch2H1 was purified from cell supernatant by protein A affinity chromatography (Pierce, Rockford, Ill.).
C. neoformans strain 24067 was obtained from the American Type Culture Collection. Other strains used in this study were J9A, J11, CN 110, H99, 62066, NIH 34, and CN 15. All strains were maintained in a suspension of 50% sterile glycerol at −80°C. Capsular GXM was purified as described previously (6). The ELISA for MAb binding to GXM was done as described above, except that goat anti-human IgG labeled with alkaline phosphatase (Sigma Chemical Co., St. Louis, Mo.) was used as a secondary reagent (5). The ability of m2H1 and ch2H1 to compete for GXM binding was studied by ELISA in experiments like those used in the past to study antibody specificity (4). Immunofluorescence and agglutination assays were done as described previously (7). Phagocytosis assays were done with the J774.16 cell line as described previously (15). The phagocytic index represents the number of ingested and attached yeast cells divided by the number of macrophages. The ability of ch2H1 to elicit acute lethal toxicity was determined in Swiss Webster mice (Charles River Laboratories, Inc., Wilmington, Mass.) infected with 5 × 105 yeast cells (strain 24067) via tail vein injection as described previously (12, 16). Eight days after infection, the mice were bled from the retroorbital plexus. Their serum was analyzed to determine the concentration of cryptococcal polysaccharide antigen, and a baseline hematocrit was obtained. Mice were placed into two experimental groups and matched for approximately equivalent serum polysaccharide levels. On the 10th day following infection, the mice were given 0.5 mg of antibody through the tail vein. The endpoint to determine toxicity was death, with a secondary endpoint being observed signs of illness, such as ataxic gait or shivering. These clinical signs have been observed in prior experiments with antibody-mediated acute lethal toxicity (ALT) (12, 13).
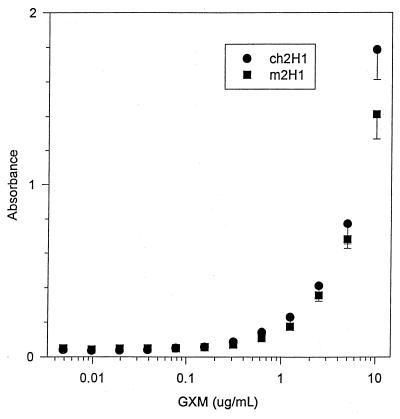
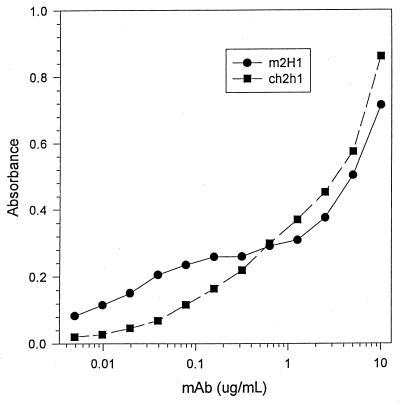
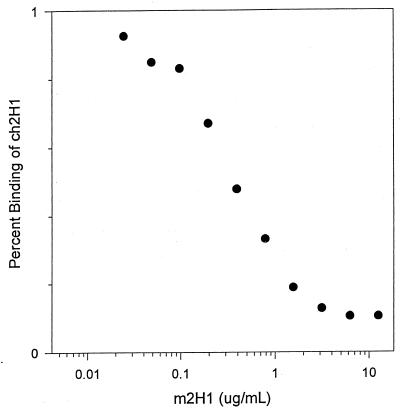
Mouse-human chimeric antibodies retain the murine antigen binding site and, on this basis, should retain the same specificity. However, constant region interactions can affect antigen binding (8, 9), and we compared the binding of m2H1 to that of ch2H1 by using several serological assays, including two ELISAs, an agglutination assay, and indirect immunofluorescence. One ELISA measured the binding of different concentrations of antibody to a constant amount of GXM attached to polystyrene (Fig. 1). The second assay measured the binding of a constant antibody concentration to a plate coated with various amounts of GXM (Fig. 2). Although minor differences in antibody binding as denoted by absorbance readings were measured at high and low antibody and antigen concentrations in the ELISAs, both antibodies exhibited similar binding characteristics. The competition assay revealed that m2H1 inhibited the binding of ch2H1 (Fig. 3). The agglutination endpoints of ch2H1 and m2H1 for C. neoformans cells were 3.125 (1:32) and 1.56 (1:64) μg/ml, respectively, which are within the error of the measurement. Both ch2H1 and m2H1 produced identical annular indirect immunofluorescence patterns when bound to a panel of C. neoformans strains (data not shown). Hence, ch2H1 and m2H1 displayed very similar binding characteristics by the four serological assays.
FIG. 1.
Binding of m2H1 and ch2H1 to GXM by ELISA under conditions in which the antibody concentration is held constant (1 μg/ml) and the amount of GXM added to the well is varied as shown.
FIG. 2.
Binding of m2H1 and ch2H1 to GXM by ELISA under conditions in which the GXM added to the well is held constant (1 μg/ml) and the antibody concentration is varied as shown.
FIG. 3.
Murine MAb 2H1 inhibits the binding of ch2H1 to GXM. For this experiment, the chimeric antibody concentration was held constant (1 μg/ml), while the murine MAb m2H1 concentration was varied as shown.
To determine whether the constant region in the ch2H1 antibody was functional, we tested its ability to promote phagocytosis with a macrophage-like cell line. m2H1 is known to be a potent opsonin for C. neoformans, but there are no data available for the efficacy of the human IgG2 constant region against this fungus. Both m2H1 and ch2H1 significantly enhanced the uptake of yeast cells by the J774.16 cells. The P values for m2H1 and ch2H1 compared to those for cells with no antibody added were both P < 0.001, with a 95% confidence interval (Student’s t test.) The opsonic efficacy of m2H1 was significantly better than that of ch2H1 (P = 0.004, 95% confidence interval).
Administration of murine IgG1 MAb to Swiss Webster mice chronically infected with C. neoformans can produce acute lethal toxicity (16). The administration of ch2H1 to mice during the acute stage of infection with C. neoformans produced no toxicity. All four mice receiving ch2H1 survived, and none displayed abnormal behaviors associated with ALT, such as ataxic gait and shivering (12). In contrast, all mice receiving m2H1 exhibited toxicity, and three of six mice died within 1 h of antibody injection (12). Their symptoms preceding death were similar to the previously described onset of ALT (12, 13).
The effort to create chimeric antibodies is driven primarily by an effort to reduce the toxicity and increase the efficacy of murine antibodies. A toxicity particular to efforts to develop antibody therapy for C. neoformans infection is demonstrated by the fact that administration of antibody to mice with chronic infection can lead to ALT (16). ALT results from the release of platelet-activating factor as a consequence of Fc receptor cross-linking (13). ALT is associated with certain murine isotypes (12, 13), and there is currently no information on whether human constant regions mediate this effect. ch2H1 did not cause ALT in our experiment, while the mice receiving m2H1 had a 50% mortality rate. This experiment highlights the critical role of the constant region in the ALT effect and indicates that human IgG2, like murine IgG3 (12), does not cause this effect.
In summary, we report the construction of a chimeric MAb derived from the murine MAb m2H1. The antibodies had similar binding patterns to the capsular polysaccharide of C. neoformans. Both were potent opsonins of yeast cells in an in vitro experiment with murine macrophages. Unfortunately, the ch2H1 cell line is a low antibody producer, and we did not obtain sufficient amounts of protein to investigate its protective efficacy. These results indicate that both the antigen binding site and the constant region of the ch2H1 are functional. The absence of toxicity in mice suggests a potential advantage to the human IgG2 isotype over murine IgG1.
Acknowledgments
A.C. is supported by NIH awards AI33774, AI3342, and HL-59842-01 and is a recipient of a Burroughs Wellcome Development Therapeutics Award.
REFERENCES
- 1.Casadevall, A. 1993. Cryptococcosis: the case for immunotherapy. Cliniguide Fungal Infect. 4:1–5. [Google Scholar]
- 2.Casadevall, A. 1999. Passive antibody therapies: progress and continuing challenges. Clin. Immunol. 93:5–15. [DOI] [PubMed] [Google Scholar]
- 3.Casadevall, A., W. Cleare, M. Feldmesser, A. Glatman-Freedman, D. L. Goldman, T. R. Kozel, N. Lendvai, J. Mukherjee, L.-A. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to Cryptocococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casadevall, A., J. Mukherjee, S. J. N. Devi, R. Schneerson, J. B. Robbins, and M. D. Scharff. 1992. Antibodies elicited by a Cryptococcus neoformans glucuronoxylomannan-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J. Infect. Dis. 65:1086–1093. [DOI] [PubMed] [Google Scholar]
- 5.Casadevall, A., J. Mukherjee, and M. D. Scharff. 1992. Monoclonal antibody ELISAs for cryptococcal polysaccharide. J. Immunol. Methods 154:27–35. [DOI] [PubMed] [Google Scholar]
- 6.Cherniak, R., L. C. Morris, B. C. Anderson, and S. A. Meyer. 1991. Facilitated isolation, purification, and analysis of glucuronoxylomannan of Cryptococcus neoformans. Infect. Immun. 59:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleare, W., S. Mukherjee, E. D. Spitzer, and A. Casadevall. 1994. Prevalence in Cryptococcus neoformans strains of a polysaccharide epitope which can elicit protective antibodies. Clin. Diagn. Lab. Immunol. 1:737–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper, L. J. N., A. R. Schickman, D. D. Glass, D. Kangisser, M. W. Cunningham, and N. S. Greenspan. 1993. Role of heavy chain constant domains in antibody-antigen interaction. J. Immunol. 150:2231–2242. [PubMed] [Google Scholar]
- 9.Cooper, L. J. N., J. C. Schimenti, D. D. Glass, and N. S. Greenspan. 1991. H chain C domains influence the strength of binding of IgG for streptococcal group A carbohydrate. J. Immunol. 146:2659–2663. [PubMed] [Google Scholar]
- 10.Currie, B. P., and A. Casadevall. 1994. Estimation of the prevalence of cryptococcal infection among HIV infected individuals in New York City. Clin. Infect. Dis. 19:1029–1033. [DOI] [PubMed] [Google Scholar]
- 11.Fleuridor, R., Z. Zhong, and L. Pirofski. 1998. A human IgM monoclonal antibody prolongs survival of mice with lethal cryptococcosis. J. Infect. Dis. 178:1213–1216. [DOI] [PubMed] [Google Scholar]
- 12.Lendvai, N., and A. Casadevall. 1999. Antibody mediated toxicity in Cryptococcus neoformans infection: mechanism and relationship to antibody isotype. J. Infect. Dis. 180:791–801. [DOI] [PubMed] [Google Scholar]
- 13.Lendvai, N., X. Qu, W. Hsueh, and A. Casadevall. 2000. Mechanism for the isotype dependence of antibody-mediated toxicity in Cryptococcus neoformans infected mice. J. Immunol. 164:4367–4374. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 60:4534–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee, S., M. Feldmesser, and A. Casadevall. 1996. J774 murine macrophage-like cell interactions with Cryptococcus neoformans in the presence and absence of opsonins. J. Infect. Dis. 173:1222–1231. [DOI] [PubMed] [Google Scholar]
- 16.Savoy, A. C., D. M. Lupan, P. B. Mananlo, J. S. Roberts, A. M. Schlageter, L. C. Weinhold, and T. R. Kozel. 1997. Acute lethal toxicity following passive immunization for treatment of murine cryptococcosis. Infect. Immun. 65:1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spitzer, E. D., S. G. Spitzer, L. F. Freundlich, and A. Casadevall. 1993. Persistence of the initial infection in recurrent cryptococcal meningitis. Lancet 341:595–596. [DOI] [PubMed] [Google Scholar]
- 18.Vecchiarelli, A., and A. Casadevall. 1998. Antibody-mediated effects against Cryptococcus neoformans: evidence for interdependency and collaboration between humoral and cellular immunity. Res. Immunol. 149:321–333. [DOI] [PubMed] [Google Scholar]
- 19.Yuan, R., A. Casadevall, G. Spira, and M. D. Scharff. 1995. Isotype switching from IgG3 to IgG1 converts a non-protective murine antibody to C. neoformans into a protective antibody. J. Immunol. 154:1810–1816. [PubMed] [Google Scholar]
- 20.Yuan, R. R., G. Spira, J. Oh, M. Paizi, A. Casadevall, and M. D. Scharff. 1998. Isotype switching increases antibody protective efficacy to Cryptococcus neoformans infection in mice. Infect. Immun. 66:1057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zebedee, S. L., R. K. Koduri, J. Mukherjee, S. Mukherjee, S. Lee, D. F. Sauer, M. D. Scharff, and A. Casadevall. 1994. Mouse-human immunoglobulin G1 chimeric antibodies with activity against Cryptococcus neoformans. Antimicrob. Agents Chemother 38:1507–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong, Z., and L.-A. Pirofski. 1998. Antifungal activity of a human antiglucuronoxylomannan antibody. Clin. Diagn. Lab. Immunol. 5:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]