Abstract
To clarify what bacterial species of commensal intestinal microbes are recognized as the antigens that induce a serum antibody response in patients with inflammatory bowel disease (IBD), 72 subjects consisting of 12 Crohn’s disease patients, 30 ulcerative colitis patients, and 30 healthy volunteers were examined for their titers of serum antibody to these intestinal bacteria. In IBD patients, as a result, significant elevations of both the immunoglobulin G (IgG) and IgA titers to Bacteroides ovatus were found. Immunoblotting showed that a definite 19.5-kDa band of B. ovatus was bound to the serum antibody raised in IBD patients. It was thus concluded that B. ovatus causes serum antibody responses in IBD patients, and a 19.5-kDa molecule of this bacterium appears to be the responsible antigen, although the role of this event in pathogenesis remains unclear.
An increasing number of both clinical and laboratory findings support the importance of luminal bacteria in the pathogenesis of inflammatory bowel disease (IBD) (17). Actually, the anatomic sites of the intestine in which bacteria colonize with a high density, such as the distal ileum and colon, are sites that are frequently affected by inflammation in IBD patients. In addition, the use of antibiotics or a diversion of the fecal stream is found to reduce the activity of inflammation in patients with IBD, thus supporting the notion that intestinal bacteria play an important role in sustaining inflammation in IBD (21, 24). Results of studies using knockout mice which had disrupted genes for cytokines or cell surface structures for immunity further support the role of luminal microorganisms in the development of IBD. For example, knockout mice with interleukin-2 (6), interleukin-10 (23), or T-cell receptors (7) do not develop disease when they are reared in a germfree environment. However, once the normal gut microflora is restored, inflammatory disease occurs. The need for a normal intestinal microflora for the initiation and/or progression of inflammatory lesions has also been reported for HLA B27/β2 microglobulin transgenic rats (19). Among the commensal intestinal microbes colonizing both IBD patients and experimental animals, anaerobic bacteria, particularly of the Bacteroides genus, are thought to be responsible for the development of inflammation (1, 16, 19).
The elevation of the titer of serum antibody to a wide variety of antigens, including microbes, has been reported for IBD patients. An increased titer of serum agglutinins to anaerobic intestinal bacteria, especially Bacteroides vulgatus, has been found in IBD patients (2, 12, 16, 26). These findings suggest that species of the Bacteroides genus of the intestinal microflora are the organisms which tend to injure the gut tissue and thus induce inflammation accompanied by an elevation of serum antibodies to these bacteria. Based on such evidence, in this study we intended to clarify what bacterial species of intestinal microflora and which component of such species become the antigens which eventually cause a serum antibody response in IBD patients.
MATERIALS AND METHODS
Subjects.
The 72 subjects investigated in this study included 12 Crohn’s disease (CD) patients (11 males and 1 female; age range, 21 to 47 years; mean, 30.2 years), 30 ulcerative colitis (UC) patients (20 males and 10 females; age range, 15 to 65 years; mean, 33.0 years), and 30 healthy volunteers (HVs; 16 males and 14 females; age range, 22 to 56 years; mean, 29.7 years). With respect to the classification of the clinical stage, six CD patients and eight UC patients were thought to be in an active stage. The diagnoses of CD and UC were based on barium enema findings, results of endoscopies that included the whole colon and the ileocecal region, and a histopathological examination. The CD and UC patients were considered to be in the inactive stage when subjective symptoms were absent, C-reactive protein results were normal, and the test for occult blood was negative. The remaining patients were regarded as being in the active stage. Informed consent was obtained from all subjects.
Analysis of fecal flora.
Fecal floras were examined according to the method of Benno and Mitsuoka (3) using 3 nonselective agar plates and 12 selective agar plates. After incubation for 2 days (aerobes) and 3 days (anaerobes), the fecal bacteria were classified into 12 bacterial groups (Pseudomonas, Enterococcus, Enterobacterium, Staphylococcus, Corynebacterium, Lactobacillus, Bacteroides, Bifidobacterium, Eubacterium, Peptococcus, Veillonella, and Clostridium) and yeasts were classified by examinations of both colonial and cellular morphology, Gram staining, spore formation, and aerobic or anaerobic growth.
Measurement of serum antibody using whole bacterial cells.
Freshly prepared bacterial cells were suspended in 0.5% formalin and kept at room temperature overnight. After being washed with phosphate-buffered saline (PBS), 500 μl of the bacterial cell suspension (1.5 × 109/ml) was added to 5 μl of diluted serum obtained from the same individual for bacterial sampling and then they were incubated for 30 min at 4°C. Next, the cells were washed, suspended in 500 μl of PBS, and added to 1 μl of 10-times-diluted fluorescein isothiocyanate (FITC)-conjugated anti-human immunoglobulin G (IgG) (EY Laboratories Inc., San Mateo, Calif.). After incubation for 30 min at 4°C, the sample was measured by a fluorometer (Fluoroskan II; Labsystems Oy, Helsinki, Finland). The levels of antibodies bound to the cells were measured by the change in fluorescence intensity (the fluorescence intensity in the presence of FITC-conjugated anti-human IgG − the fluorescence intensity in the absence of FITC-conjugated anti-human IgG).
Measurement of serum antibody by ELISA.
The titers of IgG and IgA class antibodies reacting with bacteria in serum were measured by enzyme-linked immunosorbent assay (ELISA). To prepare the antigen for ELISA, bacteria suspended in PBS (∼108 CFU/ml) were disrupted by sonication using a sonicator (Biorupture; CosmoBio Inc., Tokyo, Japan) at a high setting for 30 min. To ensure a reproducibility in ELISA, the bacterial sonicate that contained more than 10 μg of protein/ml was used as the coating antigen. Bacteroides ovatus (JCM 5824) and B. vulgatus (JCM 5826) were provided by the Japan Collection of Microorganisms, Wako, Saitama, Japan. For the assay, 50 μl of bacterial lysate (10 μg of protein/ml in carbonate-bicarbonate coating buffer) was added to a 96-well microtiter plate and kept at 4°C overnight. The plate was then washed with PBS containing 0.05% (vol/vol) Tween 20 and blocked with 10% (wt/vol) bovine serum albumin in PBS for 2 h at room temperature. Next, serum samples were serially diluted in PBS-Tween 20, and 50 μl of each was loaded in duplicate onto the plate. After incubation for 2 h at room temperature, the plate was washed and then 50 μl of the peroxidase (1 μg/ml)-conjugated goat IgG fraction raised against human IgG or peroxidase-conjugated goat IgG fraction raised against human IgA (ICN Pharmaceuticals, Inc., Aurola, Ohio) was added to each well. The plate was then incubated for a further 2 h. The conjugated enzyme was detected by the addition of 0-phenylenediamine dihydrochloride, and the absorbance in each well was read at 495 nm using a plate reader. The absorbance reading was obtained from an appropriate dilution of serum whose titer was within the range in which a linear dose-absorbance relationship was available. The antibody titer of the sample was calculated by comparing it with that of a standard serum sample obtained from a CD patient (patient 1), whose titer to each bacterial species was arbitrarily assigned 1,000 ELISA units. The data were statistically analyzed by using Student’s t test for unpaired data (two tailed). An associated probability of <0.05 was considered to be statistically significant.
Immunoblot analysis.
Immunoblotting was done according to a previously reported method (14). Briefly, bacterial lysate was mixed with concentrated sample buffer at five times the normal concentration (5% sodium dodecyl sulfate, 50% glycerol, 250 mM Tris-HCl, 0.1% bromophenol blue, 15% 2-mercaptoethanol [2-ME]) and boiled for 5 min. The samples were then electrophoresed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto a polyvinylidine fluoride membrane (Immobilon-P; Millipore Corp., Bedford, Mass.). The membrane was blocked in PBS containing 5% nonfat dried milk for 2 h, reacted with human serum diluted to 200-fold in PBS-0.1% Tween 20, and incubated with peroxidase-conjugated protein A (Amersham, Little Chalfont, Buckinghamshire, United Kingdom). After being washed with PBS-0.1% Tween 20, the membrane was developed using the enhanced-chemiluminescence system.
16S rDNA sequence and construction of the phylogenetic tree.
The 16S ribosomal DNAs (rDNAs) of the TS2 strain were amplified by PCR with the universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′; positions 8 to 27 in Escherichia coli 16S rRNA) and 1492R (5′ -GGTTACCTTGTTACGACTT-3′; positions 1510 to 1492). Amplification was carried out with a DNA thermal cycler (model MP; TAKARA, Kyoto, Japan) according to the following program: 95°C for 3 min, followed by 30 cycles consisting of 95°C for 0.5 min, 60°C for 0.5 min, and 72°C for 1.5 min, with a final extension period at 72°C for 10 min. The amplified 16S rDNAs were purified by using an UltraClean PCR Clean-up DNA purification kit (MO BIO Laboratories, Solana Beach, Calif.). Cycle sequencing reactions were performed using an ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Foster City, Calif.). An ABI PRISM 310 genetic analyzer (Applied Biosystems) was used for sequence determination. The previously determined 16S rRNA sequences used for comparisons in this study were retrieved from the DDBJ, EMBL, and GenBank nucleotide sequence databases. Sequence data were aligned with the CLUSTAL W program (25) and corrected by manual inspection. The nucleotide substitution rates (Knuc values) were calculated (11) after gaps and unknown bases were eliminated. The phylogenetic tree was constructed by the neighbor-joining method (22). A bootstrap resampling analysis (10) was performed to estimate the degree of confidence of the tree topologies.
RESULTS
Level of serum antibody to commensal bacterial flora.
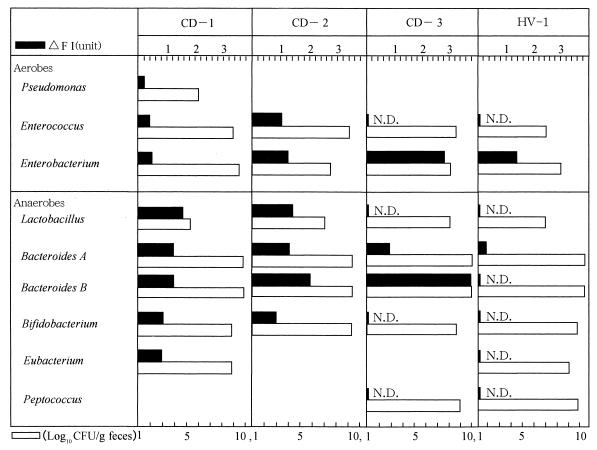
To preliminarily examine the level of serum antibody to the autologous intestinal bacterial flora, one and three subjects were selected out of the HV and CD groups, respectively, because these CD patients were in the active stage during their stay in our hospital; thus, their sera were expected to contain a large amount of antibody specific to autologous intestinal bacteria. Using feces specimens from these subjects, the numbers of fecal bacteria belonging to each bacterial group were determined (Fig. 1, open bars) and a bacterial species which could be isolated most frequently from each bacterial group was used as the antigenic bacterial cell to which serum antibody binds. Since the population size of the Bacteroides group was the largest in the fecal flora, both the most frequently isolated species, which was arbitrarily named Bacteroides A, and the next most frequently isolated species, which was named Bacteroides B, were selected for this assay. In the groups of aerobes, Enterobacterium was the predominant bacterial group obtained from CD patients (CD-1, CD-2, CD-3), and a high level of serum antibody to a species of this group was observed in patient CD-3.
FIG. 1.
Counts of fecal bacteria in the bacterial group and levels of serum antibody to the bacteria. The number of bacteria belonging to each bacterial group in the feces (open bars) and the autologous titer of serum antibody to the bacterial species (filled bars) which is most frequently isolated in each bacterial group were measured in CD-1, CD-2, CD-3, and HV-1 by the method discussed in Materials and Methods. N.D., not detected. Δ FI, change in fluorescence intensity.
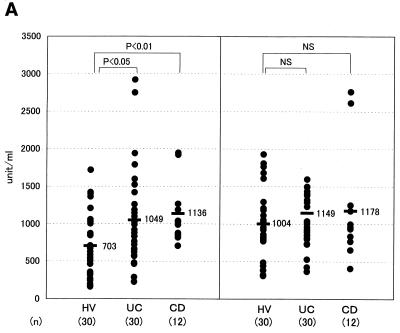
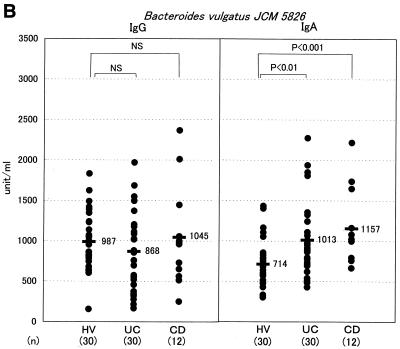
This species was revealed to be E. coli by bacteriological examinations. Among the groups of anaerobes, Bacteroides was the bacterial group whose number was the highest in these CD patients, and we found a high titer of serum antibody, especially to the Bacteroides B species. The Bacteroides B strain obtained from CD-3 exhibited the highest level of antibody binding and was named TS2. Interestingly, all of the Bacteroides B strains, including strain TS2 obtained from those three CD patients, were found to be the same species based on an analysis using an API 20A kit (Japan bioMerieux, Tokyo, Japan). To examine the titers of serum antibody to TS2 in the population of IBD patients, the sera obtained from these patients were assayed by ELISA with TS2 as the antigen (Fig. 2A). The specific IgG levels in CD and UC patients were significantly higher than the levels observed in the HVs (P < 0.01 and P < 0.05, respectively), although the specific IgA antibody levels were not significantly different between IBD patients and HVs. When B. vulgatus was used as the ELISA antigen, however, no significant elevation of the IgG antibody was found in CD or UC patients compared with the level in HV subjects (Fig. 2B). On the other hand, the levels of IgA in CD and UC patients were higher than those of IgA in the HV subjects.
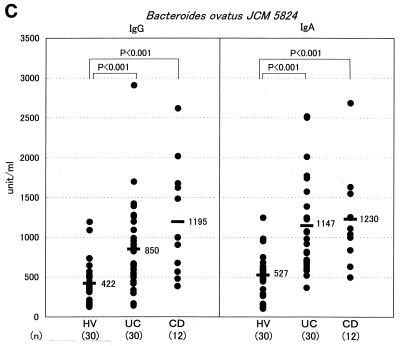
FIG. 2.
Measurement of the serum antibody titer to Bacteroides by ELISA in the IBD populations. Titers of IgG and IgA class antibodies reacting with Bacteroides strain TS2 (A), B. vulgatus JCM 5826 (B), and B. ovatus JCM 5824 (C) were measured by ELISA in the sera of HV, UC, and CD subjects. Each dot represents the mean value for each subject from triplicate samples. A horizontal bar with a number on its right represents the mean value for each population. NS, not significant.
B. vulgatus was thus suggested to be sufficiently antigenic to induce a mucosal IgA antibody response but not so as to cause a systemic IgG antibody response in IBD patients. No significant elevation in the IgG antibody level to E. coli or lactobacilli was found in the IBD patients based on the findings of this assay (data not shown). Staphylococcus, Corynebacterium, or Clostridium was undetectable in the feces of these CD patients. Yeasts and Veillonella spp. were detectable only in CD-2 and CD-1, respectively. Therefore, an examination of levels of serum antibodies to these five groups of intestinal microbes was not done.
Identification of the species name for TS2.
To know the exact species name for Bacteroides TS2, TS2 was examined by 16S rDNA sequence analysis. This resulted in a phylogenetic tree in which the TS2 branch was constructed (data not shown). According to this analysis, Bacteroides TS2 was found to be closest to Bacteroides thetaiotaomicron, B. ovatus, and Bacteroides fragilis, in that order. An analysis by Analytab Products enzyme substrate tests showed TS2 to be almost identical to B. ovatus; however, it was substantially different from B. thetaiotaomicron. For example, esterase C4 activity was weakly positive in both TS2 and B. ovatus JCM 5824 but negative in B. thetaiotaomicron JCM 5827. Moreover, leucine acrylamidase activity was negative in both TS2 and B. ovatus but positive in B. thetaiotaomicron. Based on these findings, we identified TS2 as B. ovatus.
Characterization of the B. ovatus antigen recognized by serum antibody in IBD patients.
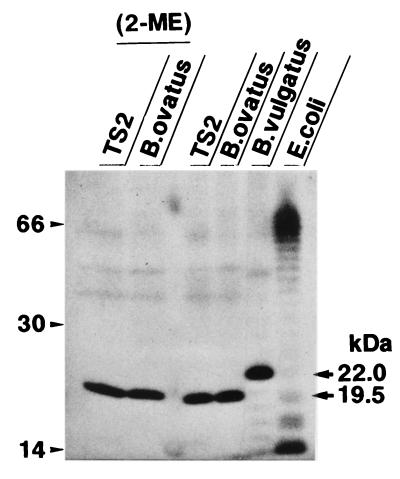
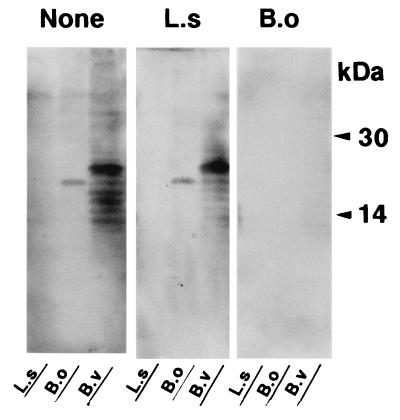
Since TS2 was identified to be B. ovatus, the level of specific antibody in serum to this bacterial species in IBD patients was examined by ELISA using B. ovatus JCM 5824, a standard strain of B. ovatus. As a result, the levels of specific IgG antibodies in CD and UC patients were approximately from three (P < 0.001) to two (P < 0.001) times higher than the level of IgG in HVs, respectively (Fig. 2C). In addition, the levels of specific IgA antibody in these CD and UC patients were also two times higher (P < 0.001) than the level of IgA in HVs. Thus, B. ovatus was again shown to be one of the predominant commensal intestinal bacterial species that causes a systemic antibody response in IBD patients. With regard to the relationship between disease activity and the IgG level, there was no significant difference by statistical analysis between CD patients (active stage, n = 6; IgG level [mean ± standard deviation], 1,340 ± 893 U per ml; inactive stage, n = 6; IgG level, 1,066 ± 493 U per ml) and UC patients (active stage, n = 8; IgG level, 1,126 ± 788 U per ml; inactive stage, n = 22; IgG level, 749 ± 427 U per ml). To characterize the antigenic epitope of B. ovatus to which the serum antibody of IBD patients binds, an immunoblot analysis was done using the serum from a CD patient (patient 2), which exhibited a high titer of antibody to both B. ovatus (2,908 U/ml) and B. vulgatus (1,372 U/ml) in ELISA. As a result, a definite 19.5-kDa band was exclusively demonstrated when the whole bacterial lysate from B. ovatus as well as TS2 was loaded on a gel for immunoblotting, while a 22.0-kDa band was shown when lysate from B. vulgatus was loaded on the gel (Fig. 3). Pretreatment of the lysate by 2-ME had no effect on the mobility of the 19.5-kDa band of B. ovatus, thus suggesting that the 19.5-kDa band consists of a single molecule. Such exclusive detection of a 19.5-kDa band in immunoblotting was found when the serum samples from 1 out of 10 HVs, 8 out of 10 UC subjects, and 9 out of 10 CD subjects were used (data not shown). The other serum samples with which we were unable to detect a 19.5-kDa band showed no distinct band or smear pattern. We next examined whether both the antigenic epitopes expressed on the 19.5-kDa molecule and the 22.0-kDa molecule cross-reacted with each other in immunoblotting with a serum which underwent absorption treatment with whole bacterial cells (Fig. 4). The treatment of the serum with Lactobacillus salivarius, one of the predominant species in the Lactobacillus group involved in the feces from patient CD-1, had very little effect on the densities of these two major bands on the film. On the contrary, treating this serum with B. ovatus rendered the serum incapable of detecting these two bands, thus indicating the cross-reaction between the antigenic epitopes on these 19.5- and 22.0-kDa molecules.
FIG. 3.
Immunoblotting using whole bacterial lysates and the serum from an IBD patient. The whole bacterial lysate obtained from TS2, B. ovatus JCM 5824, B. vulgatus JCM 5826, or E. coli JCM 1649 was loaded in each lane as shown at the top. In the two lanes on the far left, the lysates were treated by 2-ME before being loaded onto the gel. The numbers indicated in the left margin of the gel represent the molecular masses (in thousands) as determined by the mobilties of the molecular mass markers. The serum from a CD patient (patient 2) was used as an antibody for the reaction.
FIG. 4.
Treatment of serum by absorption with whole bacterial cells before being used in immunoblotting. Serum from patient 2 was treated by absorption using whole bacterial cells of L. salivarius (L.s) or B. ovatus (B.o) as indicated at the top of the figure. The serum without such treatment was used as a control (None). Then the serum was reacted with a filter to which whole bacterial lysates from L. salivarius, B. ovatus, and B. vulgatus (B.v) were loaded as indicated at the bottom of the figure.
DISCUSSION
Alterations in the fecal floras of IBD patients have been reported, but no consistent alterations in the balance or composition of the fecal floras have yet been identified. An investigation of the rectal-mucosa-associated associated bacterial floras in UC patients showed both the highest bacterial counts and the highest isolation frequency to be found for B. vulgatus, B. fragilis, and B. ovatus, in that order (18). The bacterial count of B. ovatus was around 100 times less than the count of B. vulgatus. In the present analysis of three CD patients, the count of B. ovatus (=Bacteroides B) was closest to that of Bacteroides species, which had the highest count, which closely correlates with the result quoted above.
According to the report by Cato and Johnson (5) regarding the characteristics of B. ovatus ATCC 8483, the bacterial cells were 1.3 to 2.0 μm in diameter and 1.6 to 5.0 μm in length, with rounded ends. B. ovatus is an anaerobic, non-spore-forming, nonmotile, and gram-negative rod. In addition,B. ovatus is considered to belong to a group of Bacteroides species bearing the similar phenotypic characteristics of B. fragilis, B. thetaiotaomicron, Bacteroides distasonis, B. vulgatus, and B. ovatus (8). TS2 identified as B. ovatus in the present study also exhibited almost the same characteristics as those reported by Cato and Johnson (5).
An increasing number of studies have demonstrated that the systemic and local immune responses against gut microflora are distorted in IBD patients (17). An exaggerated mucosal antibody response against intestinal bacterial flora has also been described for IBD patients. Brown and Lee (4) reported that the serum antibody titer against B. fragilis was elevated in IBD patients, although the characteristics of this bacterial antigen remain to be clarified. An elevation of the level of serum antibody to the Bacteroides genus was also demonstrated by Bamba et al. (2), who showed that high titers of serum IgG antibody to a 26-kDa protein of B. vulgatus were found in IBD patients. In the present study, we could also detect a protein band which closely corresponded to this molecule in the lysate of B. vulgatus by immunoblotting with serum from a CD patient. However, the titers of serum IgG antibody to B. vulgatus in the population of IBD patients were not statistically higher than the titers to this bacterium in the population of HVs, while the IgA antibody titers in the former were significantly higher than in the latter. On the other hand, the same analysis in these populations demonstrated a statistically significant elevation in the titers of both the IgG and IgA antibodies to B. ovatus in IBD patients than in the titers to this bacterium in HVs. However, the IgA response to TS2, which was identified with B. ovatus, was not elevated in IBD patients. This may be due to some differences in the bacterial phenotypes as well as antigenic epitopes. Actually, β-glucosidase activity was positive in TS2 but negative in B. ovatus JCM 5824.
Antibodies are present in the highest concentrations in blood serum, and IgG is the major antibody class in the serum. Functionally, IgG antibodies mediate a wide range of functions, including opsonization of antigens due to the binding of antigen-antibody complex and the activation of the complement system and thus are believed to play an important role in the systemic immune response. On the other hand, IgA antibodies are secreted into a variety of body fluids, including the intestinal contents, and are also widely distributed in the blood serum (9). Accumulating evidence suggests the anti-inflammatory nature of IgA; intact human IgA antibodies fail to activate the complement when they are complexed with antigen while also interfering with complement activation by IgM and IgG antibodies (13). Although IgA antibodies are principally responsible for immunity at the mucosal surface, monomeric IgA, which is distributed mainly in the serum, has been postulated to suppress the immune responses in inflammatory foci, particularly when the threat posed by a pathogen declines after its successful disposal (20). In the present study, an analysis of the sera from IBD patients demonstrated an elevation in the titers of both IgG and IgA antibodies to B. ovatus, but an elevation only in the titer of IgA antibody to B. vulgatus. Our results therefore suggest that B. ovatus is so highly antigenic that it surpasses the anti-inflammatory regulation exerted by an IgA response and thus can cause an inflammatory IgG response too. On the other hand, B. vulgatus does not appear to be sufficiently pathogenic to induce an IgG response in addition to an IgA response.
The reason why B. ovatus is more antigenic or pathogenic than B. vulgatus to gut tissue in IBD patients remains to be elucidated. According to an analysis of the biochemical characteristics of both bacterial species in the present study, the bacterial enzymes such as esterase and lipase, which are potentially hazardous to intestinal tissue, are believed to be more involved in B. ovatus than in B. vulgatus. The intestine has physical barriers to prevent antigen access into the submucosal lymphoid tissues that include intestinal mucus and the epithelial cell tight junction (9). An injury in these barriers caused by such bacterial enzymes would correlate with an increase in the antigen load in the submucosal immune apparatus, which then leads to an enhancement of the systemic IgG antibody response to such luminal intestinal bacteria as B. ovatus. Toxins such as B. fragilis toxin (15, 27) might thus be involved in an impairment of the barriers mediated by B. ovatus.
In the present study, we demonstrated a definite 19.5-kDa band in B. ovatus, which is clearly distinct from the 22.0-kDa band in B. vulgatus. Although the precise nature of this 19.5-kDa molecule of B. ovatus remains unclear, an antigenic epitope on this molecule appears to be identical to the epitope on the 22.0-kDa molecule of B. vulgatus because the serum, which was treated by absorption with bacterial cells of B. ovatus, failed to detect not only the 19.5-kDa band but also the 22.0-kDa band in immunoblotting. While the present study showed these molecules to be highly antigenic in IBD patients, whether these molecules play a directly pathogenic role in gut tissue remains to be elucidated.
REFERENCES
- 1.Auer, I. O., A. Roder, F. Wensinck, J. P. van de Merwa, and H. Schmidt. 1983. Selected bacterial antibodies in Crohn’s disease and ulcerative colitis. Scand. J. Gastroenterol. 18:217–223. [DOI] [PubMed] [Google Scholar]
- 2.Bamba, T., H. Matsuda, M. Endo, and Y. Fujiyama. 1995. The pathogenic role of Bacteroides vulgatus in patients with ulcerative colitis. J. Gastroenterol. 30:45–47. [PubMed] [Google Scholar]
- 3.Benno, Y., and T. Mitsuoka. 1992. Impact of Bifidobacterium longum on human fecal microflora. Microbiol. Immunol. 36:683–694. [DOI] [PubMed] [Google Scholar]
- 4.Brown, W. R., and E. Lee. 1974. Radioimmunological measurements of bacterial antibodies. Gastroenterology 66:1145–1153. [PubMed] [Google Scholar]
- 5.Cato, E. P., and J. L. Johnson. 1976. Reinstatement of species rank for Bacteroides fragilis, B. ovatus, B. distasonis, B. thetaiotaomicron, and B. vulgatus: designation of neotype strains for Bacteroides fragilis (Veillon and Zuber) Castellani and Chalmers and Bacteroides thetaiotaomicron (Distaso) Castellani and Chalmers. Int. J. Syst. Bacteriol. 26:230–237. [Google Scholar]
- 6.Contractor, N. V., H. Bassiri, T. Reya, A. Y. Park, D. C. Baumgart, M. A. Wasik, S. G. Emerson, and S. R. Carding. 1998. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-2-deficient mice. J. Immunol. 160:385–394. [PubMed] [Google Scholar]
- 7.Dianda, L., A. M. Hanby, N. A. Wright, A. Sebesteny, A. C. Hayday, and M. J. Owen. 1997. T cell receptor-αβ-deficient mice fail to develop colitis in the absence of microbial environment. Am. J. Pathol. 150:91–97. [PMC free article] [PubMed] [Google Scholar]
- 8.Eggerth, A. H., and B. H. Gagnon. 1933. The bacteroides of human feces. J. Bacteriol. 25:389–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elson, C. O. 2000. The immunology of inflammatory bowel disease, p.208–239. In J. B. Kirsner (ed.), Inflammatory bowel disease, 5th ed. W. B. Saunders, Philadelphia, Pa.
- 10.Felsenstein, J. 1985. Confidence limits of phylogenies: an approach using the bootstrap. Evolution 39:783–791. [DOI] [PubMed] [Google Scholar]
- 11.Kimura, M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111–120. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda, H., Y. Fujiyama, A. Andoh, T. Ushijima, T. Kajinami, and T. Bamba. 2000. Characterization of antibody responses against rectal mucosa-associated bacterial flora in patients with ulceractive colitis. J. Gastroenterol. Hepatol. 15:61–68. [DOI] [PubMed] [Google Scholar]
- 13.Mestecky, J., M. W. Russell, and C. O. Elson. 1999. Intestinal IgA: novel views on its function in the defence of the largest mucosal surface. Gut 44:2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noda, S., T. Nagata-Narumiya, A. Kosugi, S. Narumiya, C. Ra, H. Fujiwara, and T. Hamaoka. 1995. Do structural changes of T cell receptor complex occur in tumor-bearing state? Jpn. J. Cancer Res. 86:383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obiso, R. J., Jr., D. M. Lyerly, R. L. Van Tassell, and T. D. Wilkins. 1995. Proteolytic activity of the Bacteroides fragilis enterotoxin causes fluid secretion and intestinal damage in vivo. Infect. Immun. 63:3820–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onderdonk, A. B., M. L. Franklin, and R. L. Cisneros. 1981. Production of experimental ulcerative colitis in gnotobiotic guinea pigs with simplified microflora. Infect. Immun. 32:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onderdonk, A. B. 2000. Intestinal microflora and inflammatory bowel disease, p.144–152. In J. B. Kirsner (ed.), Inflammatory bowel disease, 5th ed. W. B. Saunders, Philadelphia, Pa.
- 18.Poxton, I. R., R. Brown, A. Sawyerr, and A. Ferguson. 1997. Mucosa-associated bacterial flora of the human colon. J. Med. Microbiol. 46:85–91. [DOI] [PubMed] [Google Scholar]
- 19.Rath, H. C., H. H. Herfarth, J. S. Ikeda, W. B. Grenther, T. E. Hamm, E. Balish, J. D. Taurog, R. E. Hammer, K. H. Wilson, and R. B. Sartor. 1996. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human β2 microglobulin transgenic rats. J. Clin. Investig. 98:945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell, M. W., D. A. Sibley, and E. B. Nikolova. 1997. IgA antibody as a non-inflammatory regulator of immunity. Biochem. Soc. Trans. 25:466–470. [DOI] [PubMed] [Google Scholar]
- 21.Rutgeerts, P., K. Goboes, M. Peeters, M. Hiele, F. Penninckx, R. Aerts, R. Kerremans, and G. Vantrappen. 1991. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet 338:771–774. [DOI] [PubMed] [Google Scholar]
- 22.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425. [DOI] [PubMed] [Google Scholar]
- 23.Sellon, R. K., S. Tonkonogy, M. Schultz, L. A. Dieleman, W. Grenther, E. Balish, D. M. Rennick, and R. B. Sartor. 1998. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 66:5224–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutherland, L., J. Singleton, and J. Sessions. 1991. Double-blind, placebo controlled trial of metronidazole in Crohn’s disease. Gut 32:1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tvede, M., S. Bondesen, O. H. Nielsen, and S. N. Rasmussen. 1983. Serum antibodies to Bacteroides species in chronic inflammatory bowel disease. Scand. J. Gastroenterol. 18:783–789. [DOI] [PubMed] [Google Scholar]
- 27.Van Tassell, R. L., D. M. Lyerly, and T. D. Wilkins. 1992. Purification and characterization of an enterotoxin from Bacteroides fragilis. Infect. Immun. 60:1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]