Abstract
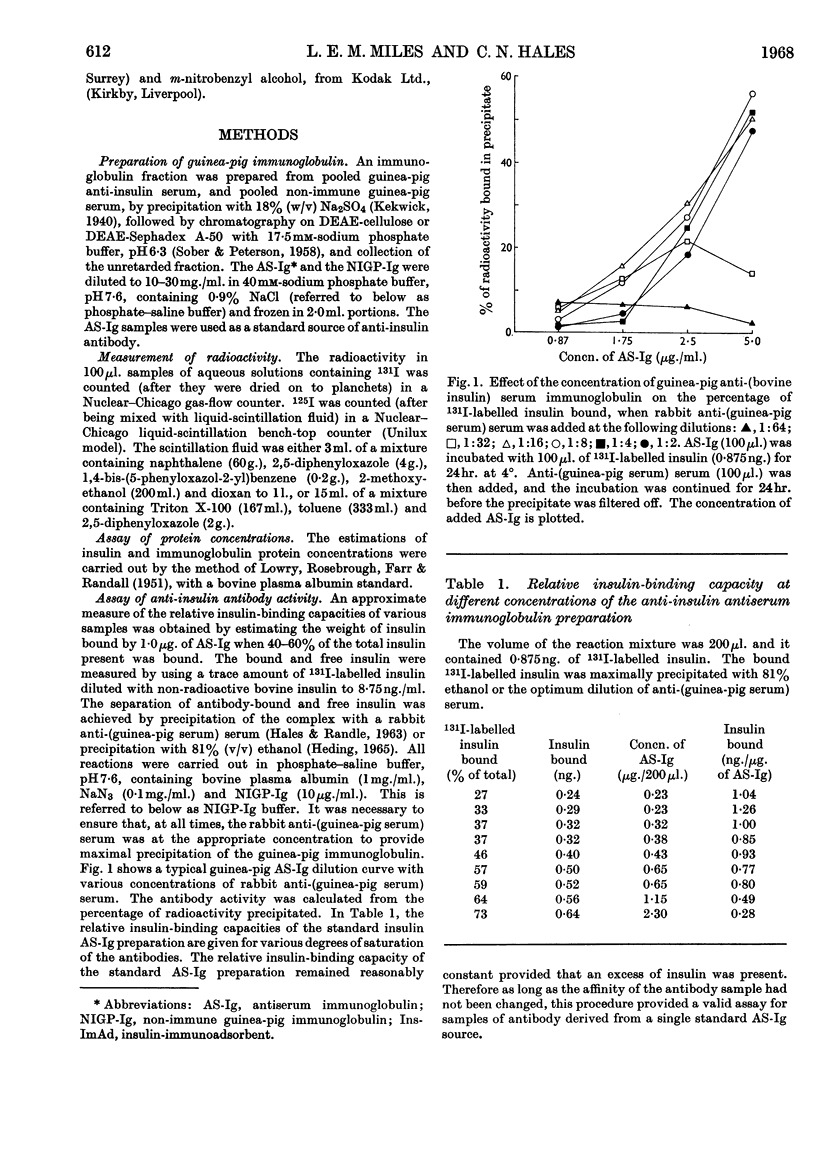
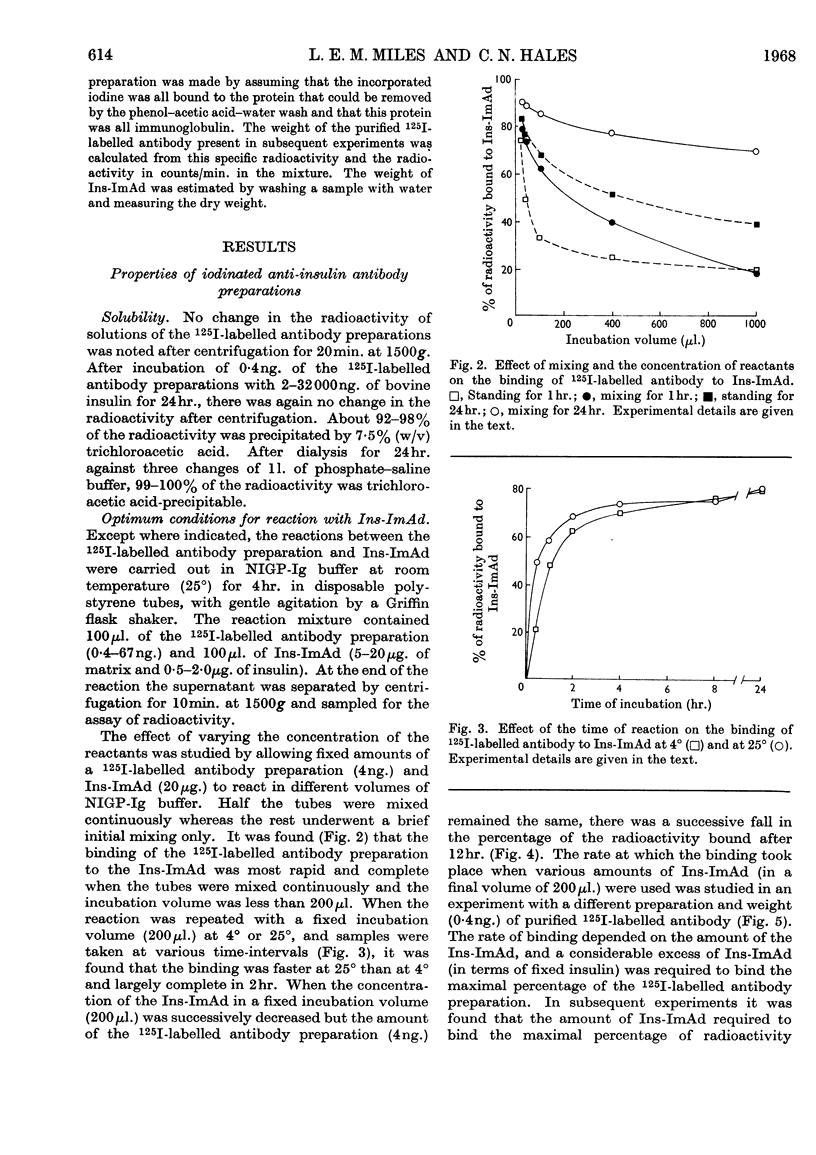
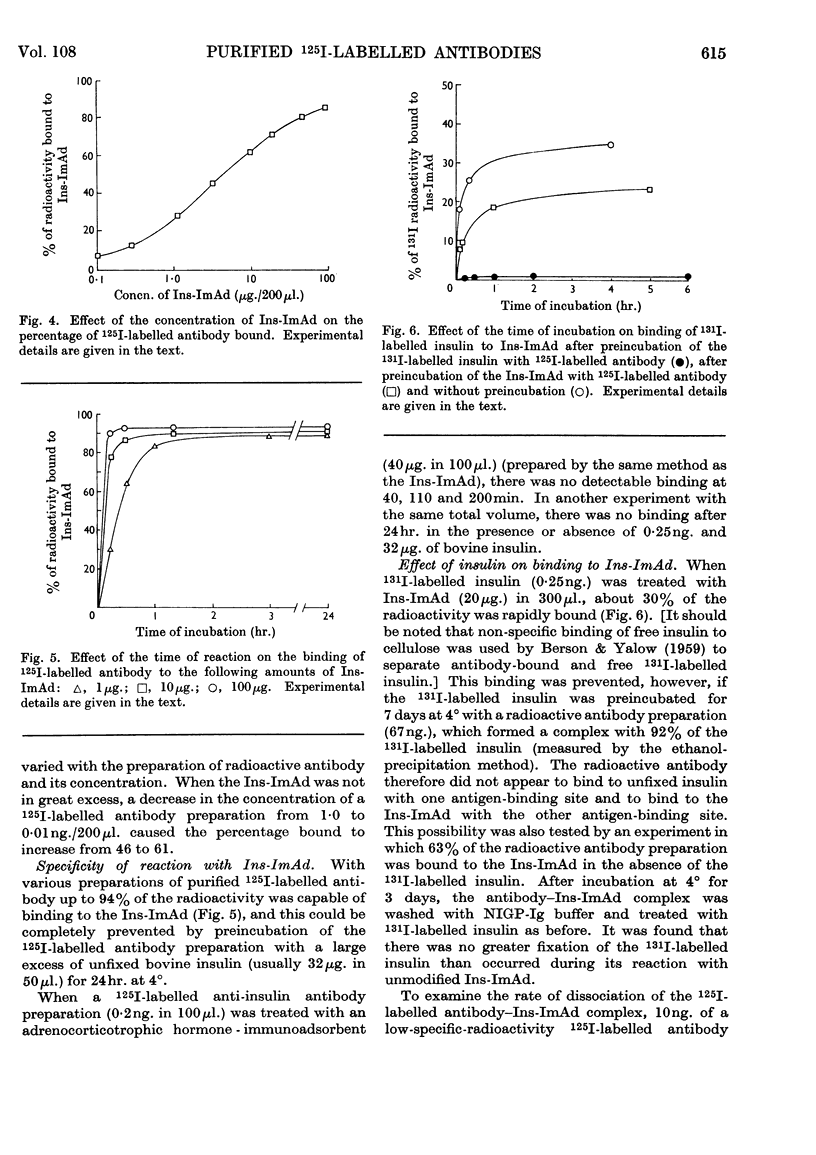
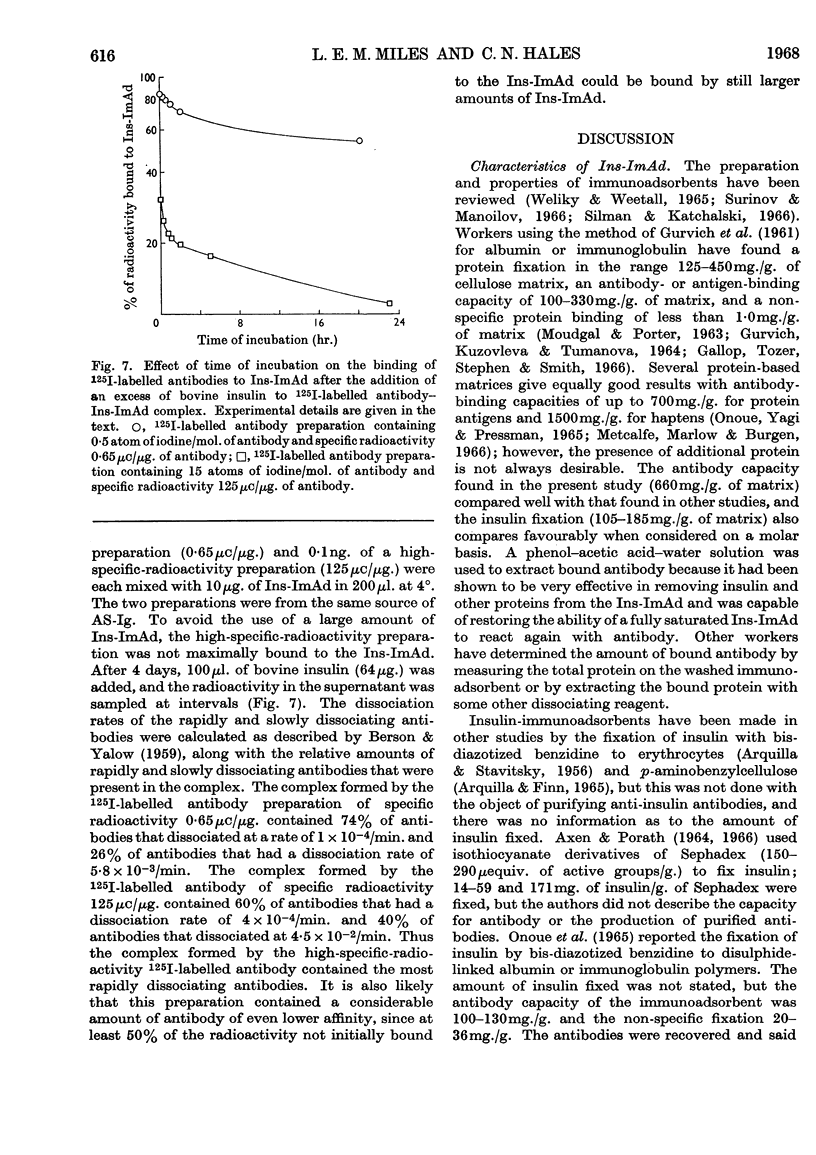
1. A procedure is described for the preparation of an insulin-immunoadsorbent containing 105–185mg. of insulin/g. of matrix, and with an antibody-binding capacity of 660mg./g. of insulin-immunoadsorbent. 2. Purified non-precipitating 125I-labelled antibodies were prepared by iodination of the antibodies while they were isolated on the insulin-immunoadsorbent in order to protect at least one antigen-binding site. 3. The radioactive antibody was simply and rapidly separated from other radioactive material and damaging reagents, and found to be up to 94% pure as judged by its reaction with insulin-immunoadsorbent and unfixed insulin. 4. The 125I-labelled antibody preparations had iodine contents of 0·5–64atoms/mol. of protein and specific radioactivities of 0·3–125mc/mg. of protein. 5. It is suggested that this is a simple method of producing a useful reagent.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARQUILLA E. R., STAVITSKY A. B. The production and identification of antibodies to insulin and their use in assaying insulin. J Clin Invest. 1956 May;35(5):458–466. doi: 10.1172/JCI103297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arquilla E. R., Finn J. Genetic control of combining sites of insulin antibodies produced by guinea pigs. J Exp Med. 1965 Oct 1;122(4):771–784. doi: 10.1084/jem.122.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axén R., Porath J. Chemical coupling of enzymes to cross-linked dextran ('Sephadex'). Nature. 1966 Apr 23;210(5034):367–369. doi: 10.1038/210367a0. [DOI] [PubMed] [Google Scholar]
- BERSON S. A., YALOW R. S. Quantitative aspects of the reaction between insulin and insulin-binding antibody. J Clin Invest. 1959 Nov;38:1996–2016. doi: 10.1172/JCI103979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERSON S. A., YALOW R. S. Radiochemical and radiobiological alterations of I 131-labeled proteins in solution. Ann N Y Acad Sci. 1957 Aug 30;70(1):56–68. doi: 10.1111/j.1749-6632.1957.tb35377.x. [DOI] [PubMed] [Google Scholar]
- GOODFRIEND T. L., LEVINE L., FASMAN G. D. ANTIBODIES TO BRADYKININ AND ANGIOTENSIN: A USE OF CARBODIIMIDES IN IMMUNOLOGY. Science. 1964 Jun 12;144(3624):1344–1346. doi: 10.1126/science.144.3624.1344. [DOI] [PubMed] [Google Scholar]
- GROSSBERG A. L., RADZIMSKI G., PRESSMAN D. Effect of iodination on the active site of several antihapten antibodies. Biochemistry. 1962 May 25;1:391–401. doi: 10.1021/bi00909a004. [DOI] [PubMed] [Google Scholar]
- Gallop R. G., Tozer B. T., Stephen J., Smith H. Separation of antigens by immunological specificity: Use of cellulose-linked antibodies as immunosorbents. Biochem J. 1966 Dec;101(3):711–716. doi: 10.1042/bj1010711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover J. S., Salter D. N., Shepherd B. P. A study of some factors that influence the iodination of ox insulin. Biochem J. 1967 Apr;103(1):120–128. doi: 10.1042/bj1030120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELMKAMP R. W., GOODLAND R. L., BALE W. F., SPAR I. L., MUTSCHLER L. E. High specific activity iodination of gamma-globulin with iodine-131 monochloride. Cancer Res. 1960 Nov;20:1495–1500. [PubMed] [Google Scholar]
- HUGHES-JONES N. C., GARDNER B., TELFORD R. THE EFFECT OF PH AND IONIC STRENGTH ON THE REACTION BETWEEN ANTI-D AND ERYTHROCYTES. Immunology. 1964 Jan;7:72–81. [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hughes-Jones N. C. Iodine-125-labelled L chains of human blood group antibodies. Nature. 1965 Aug 28;207(5000):989–990. doi: 10.1038/207989a0. [DOI] [PubMed] [Google Scholar]
- JOHNSON A., DAY E. D., PRESSMAN D. The effect of iodination on antibody activity. J Immunol. 1960 Feb;84:213–220. [PubMed] [Google Scholar]
- KATSURA T. Immunochemical studies on diphtheria antitoxin. V. Effects of iodination and acetylation on the digested antitoxin. Jpn J Exp Med. 1955 Oct;25(4-5):167–181. [PubMed] [Google Scholar]
- KOSHLAND M. E., ENGLBERGER F. M., GADDONE S. M. Identification of tyrosine at the active site of anti-p-azobenze-nearsonic acid antibody. J Biol Chem. 1963 Apr;238:1349–1352. [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOUDGAL N. R., PORTER R. R. The use of antigen-cellulose suspensions for the isolation of specific antibodies. Biochim Biophys Acta. 1963 Apr 2;71:185–187. doi: 10.1016/0006-3002(63)90998-3. [DOI] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Metcalfe J. C., Marlow H. F., Burgen A. S. Immuno-adsorbents of high capacity. Nature. 1966 Mar 12;209(5028):1142–1142. doi: 10.1038/2091142a0. [DOI] [PubMed] [Google Scholar]
- OLOVNIKOV A. M. [Preparation of immunosorbents on the basis of an emulsified nonisotactic polystyrol]. Lab Delo. 1962 Aug;8:31–34. [PubMed] [Google Scholar]
- Onoue K., Yagi Y., Pressman D. Immunoadsorbents with high capacity. Immunochemistry. 1965 Jun;2(2):181–194. doi: 10.1016/0019-2791(65)90020-0. [DOI] [PubMed] [Google Scholar]
- PRESSMAN D., ROHOLT O. Isolation of peptides from an antibody site. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1606–1610. doi: 10.1073/pnas.47.10.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESSMAN D., STERNBERGER L. A. The nature of the combining sites of antibodies; the specific protection of the combining site by hapten during iodination. J Immunol. 1951 May;66(5):609–620. [PubMed] [Google Scholar]
- ROBINSON B. H., WRIGHT P. H. Guinea-pig anti-insulin serum. J Physiol. 1961 Feb;155:302–310. doi: 10.1113/jphysiol.1961.sp006628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOBER H. A., PETERSON E. A. Protein chromatography on ion exchange cellulose. Fed Proc. 1958 Dec;17(4):1116–1126. [PubMed] [Google Scholar]
- Silman I., Katchalski E. Water-insoluble derivatives of enzymes, antigens, and antibodies. Annu Rev Biochem. 1966;35:873–908. doi: 10.1146/annurev.bi.35.070166.004301. [DOI] [PubMed] [Google Scholar]
- TERRES G., SOREM G. L. IMMUNE DEGRADATION IN PASSIVELY SENSITIZED MICE. 3. DEGRADATION OF IODINE-LABELED RABBIT ANTIBODY. J Immunol. 1965 May;94:674–681. [PubMed] [Google Scholar]
- WELIKY N., WEETALL H. H., GILDEN R. V., CAMPBELL D. H. THE SYNTHESIS AND USE OF SOME INSOLUBLE IMMUNOLOGICALLY SPECIFIC ADSORBENTS. Immunochemistry. 1964 Oct;1:219–229. doi: 10.1016/0019-2791(64)90045-x. [DOI] [PubMed] [Google Scholar]
- Weliky N., Weetall H. H. The chemistry and use of cellulose derivatives for the study of biological systems. Immunochemistry. 1965 Dec;2(4):293–322. doi: 10.1016/0019-2791(65)90031-5. [DOI] [PubMed] [Google Scholar]
- Zarrow M. X., McClintock J. A. Localization of 131-I-labelled antibody to relaxin. J Endocrinol. 1966 Dec;36(4):377–387. doi: 10.1677/joe.0.0360377. [DOI] [PubMed] [Google Scholar]


