Abstract
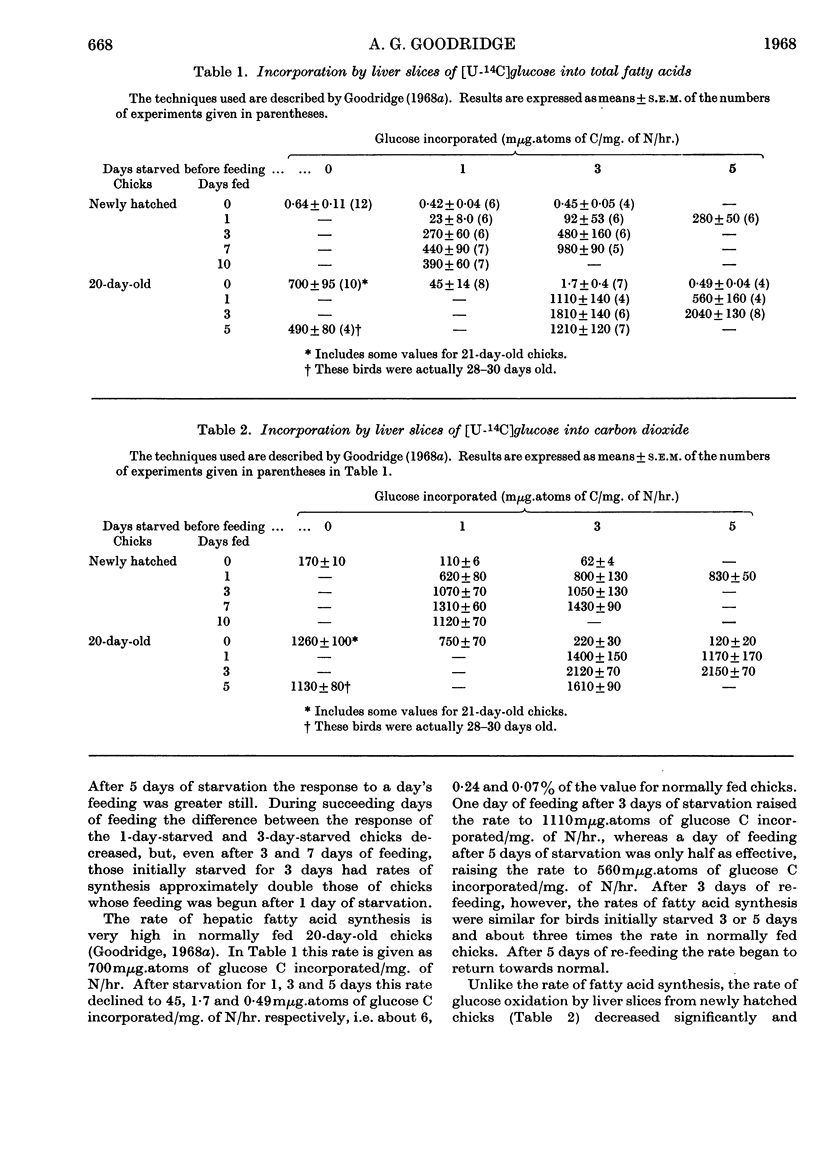
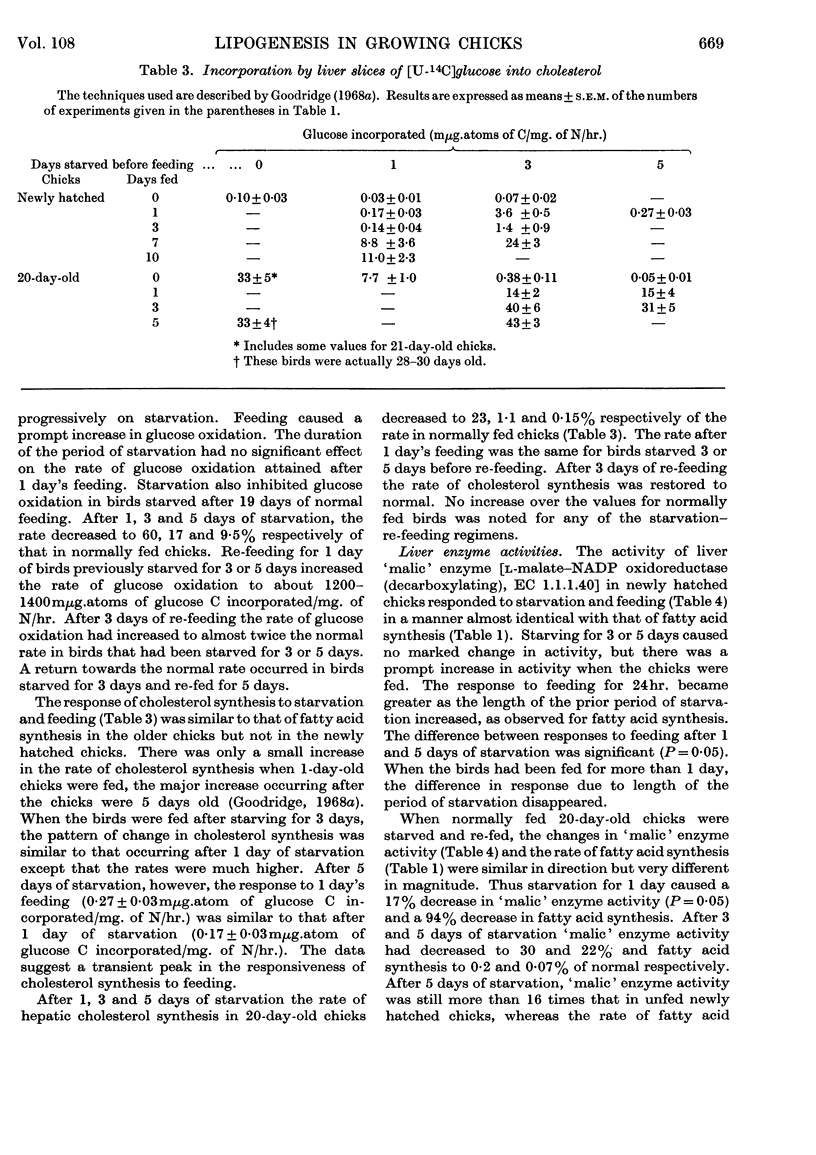
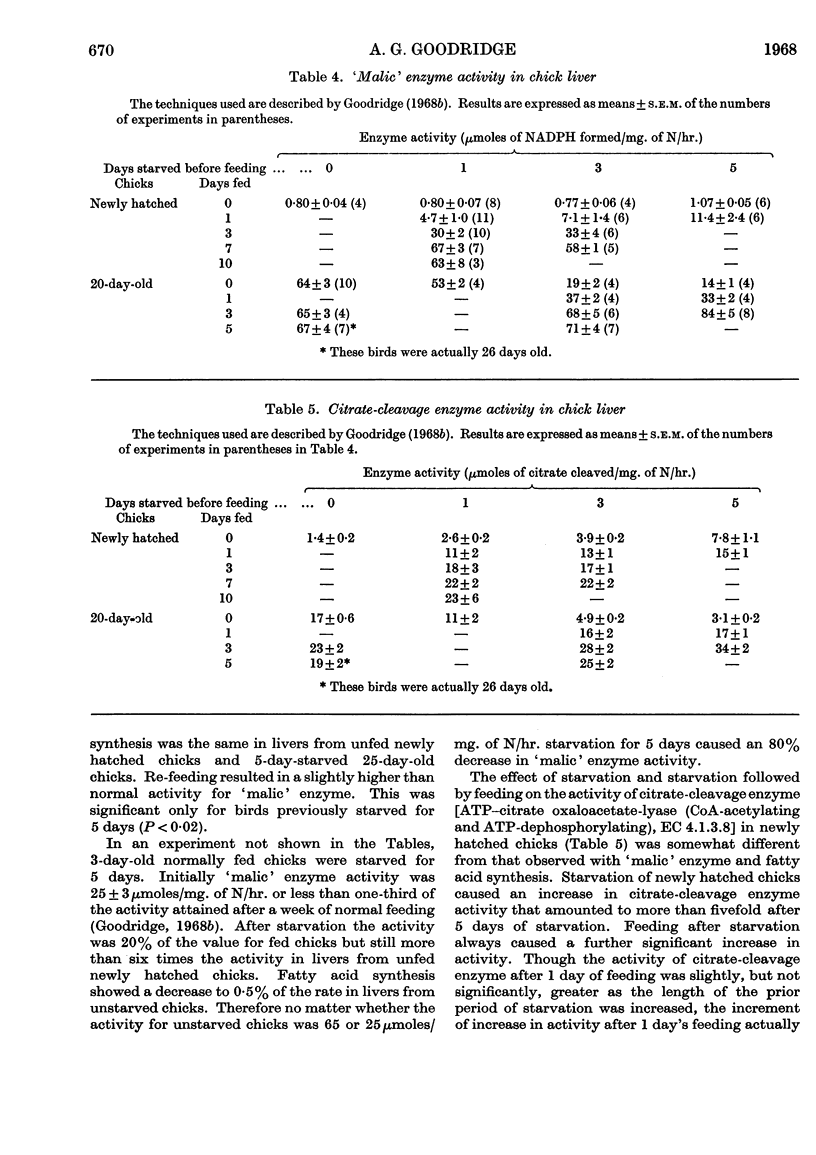
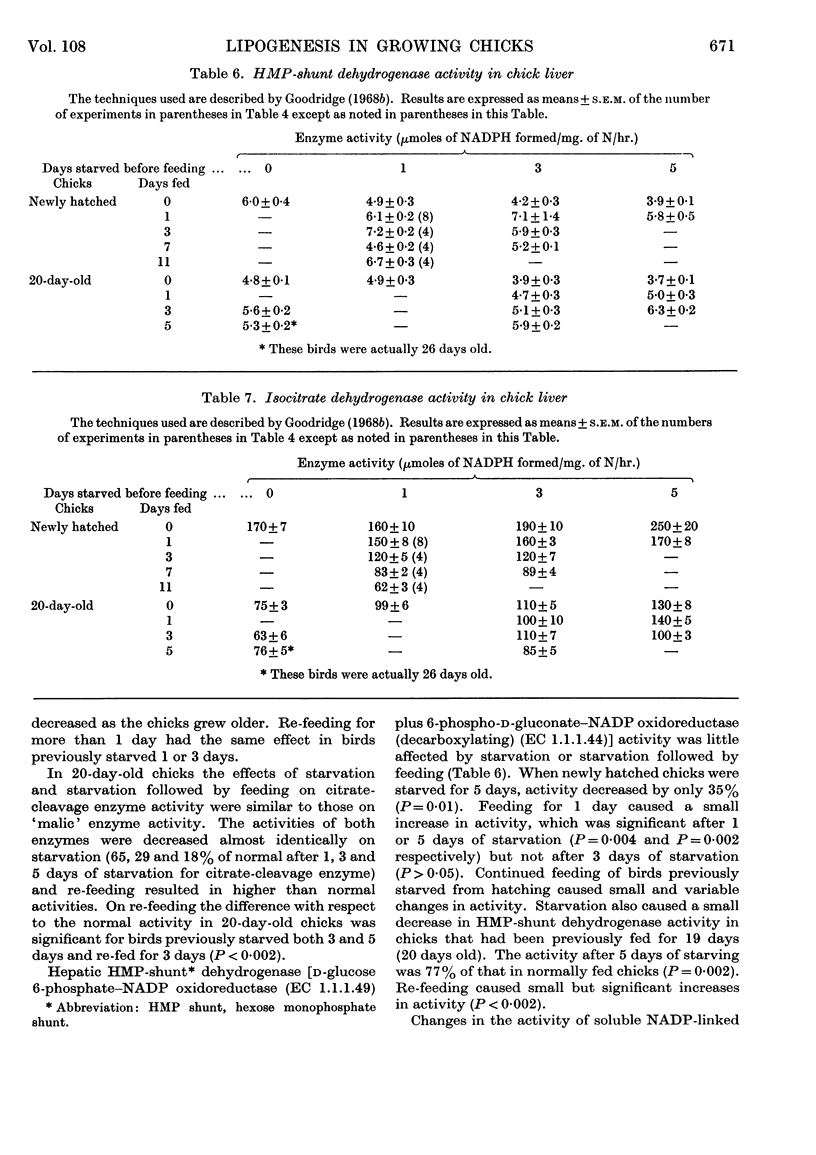
1. The conversion of [U-14C]glucose into carbon dioxide, cholesterol and fatty acids in liver slices and the activities of `malic' enzyme, citrate-cleavage enzyme, NADP-linked isocitrate dehydrogenase and hexose monophosphate-shunt dehydrogenases in the soluble fraction of homogenates of liver were measured in chicks that were starved or starved then fed. 2. In newly hatched chicks the incorporation of [U-14C]glucose and the activity of `malic' enzyme did not increase unless the birds were fed. The response to feeding of [U-14C]glucose incorporation into fatty acids increased as the starved chicks grew older. 3. Citrate-cleavage enzyme activity increased slowly even when the newly hatched chicks were unfed. On feeding, citrate-cleavage enzyme activity increased at a much faster rate. 4. In normally fed 20-day-old chicks starvation decreased the incorporation of [U-14C]glucose into all three end products and depressed the activities of `malic' enzyme and citrate-cleavage enzyme. Re-feeding increased all of these processes to normal or higher-than-normal levels. 5. In both newly hatched and 20-day-old chicks starvation increased the activity of isocitrate dehydrogenase and feeding or re-feeding decreased it. 6. Very little change in hexose monophosphate-shunt dehydrogenase activity was observed during the dietary manipulations. 7. The results indicate that increased substrate delivery to the liver is the principal stimulus to the increased rate of glucose metabolism observed in newly hatched chicks. The results also suggest that changes in the activities of `malic' enzyme and citrate-cleavage enzyme are secondary to an increased flow of metabolites through the glucose-to-fatty acid pathway and that the dehydrogenases of the hexose monophosphate shunt play a minor role in NADPH production for fatty acid synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fritz I. B., Hsu M. P. Studies on the control of fatty acid synthesis. I. Stimulation by (+)-palmitylcarnitine of fatty acid synthesis in liver preparations from fed and fasted rats. J Biol Chem. 1967 Mar 10;242(5):865–872. [PubMed] [Google Scholar]
- Goodridge A. G., Ball E. G. Lipogenesis in the pigeon: in vitro studies. Am J Physiol. 1966 Sep;211(3):803–808. doi: 10.1152/ajplegacy.1966.211.3.803. [DOI] [PubMed] [Google Scholar]
- Goodridge A. G. Citrate-cleavage enzyme, 'malic' enzyme and certain dehydrogenases in embryonic and growing chicks. Biochem J. 1968 Jul;108(4):663–666. doi: 10.1042/bj1080663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge A. G. Conversion of[U-14C]glucose into carbon dioxide, glycogen, cholesterol and fatty acids in liver slices from embryonic and growing chicks. Biochem J. 1968 Jul;108(4):655–661. doi: 10.1042/bj1080655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTCHENS T. T., VAN BRUGGEN J. T., COCKBURN R. M., WEST E. S. The effect of fasting upon tissue lipogenesis in the intact rat. J Biol Chem. 1954 May;208(1):115–122. [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. M. CITRATE AND THE CONVERSION OF CARBOHYDRATE INTO FAT. THE ACTIVITIES OF CITRATE-CLEAVAGE ENZYME AND ACETATE THIOKINASE IN LIVERS OF STARVED AND RE-FED RATS. Biochem J. 1965 Jan;94:209–215. doi: 10.1042/bj0940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornacker M. S., Ball E. G. Citrate cleavage in adipose tissue. Proc Natl Acad Sci U S A. 1965 Sep;54(3):899–904. doi: 10.1073/pnas.54.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASORO E. J., CHAIKOFF I. L., CHERNICK S. S., FELTS J. M. Previous nutritional state and glucose conversion to fatty acids in liver slices. J Biol Chem. 1950 Aug;185(2):845–856. [PubMed] [Google Scholar]
- MEDES G., THOMAS A., WEINHOUSE S. Nutritional factors in fatty acid synthesis by tissue slices in vitro. J Biol Chem. 1952 May;197(1):181–191. [PubMed] [Google Scholar]
- PANDE S. V., KHAN R. P., VENKITASUBRAMANIAN T. A. NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE-SPECIFIC DEHYDROGENASES IN RELATION TO LIPOGENESIS. Biochim Biophys Acta. 1964 Jun 15;84:239–250. doi: 10.1016/0926-6542(64)90053-8. [DOI] [PubMed] [Google Scholar]
- Swanson R. F., Curry W. M., Anker H. S. The activation of rat liver acetyl-CoA carboxylase by trypsin. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1243–1248. doi: 10.1073/pnas.58.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEPPERMAN H. M., TEPPERMAN J. The hexosemonophosphate shunt and adaptive hyperlipogenesis. Diabetes. 1958 Nov-Dec;7(6):478–485. doi: 10.2337/diab.7.6.478. [DOI] [PubMed] [Google Scholar]
- WISE E. M., Jr, BALL E. G. MALIC ENZYME AND LIPOGENESIS. Proc Natl Acad Sci U S A. 1964 Nov;52:1255–1263. doi: 10.1073/pnas.52.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYSHAK G. H., CHAIKOF I. L. Metabolic defects in the liver of fasted rats as shown by utilization of C14-labeled glucose and fructose. J Biol Chem. 1953 Feb;200(2):851–857. [PubMed] [Google Scholar]
- YOUNG J. W., SHRAGO E., LARDY H. A. METABOLIC CONTROL OF ENZYMES INVOLVED IN LIPOGENESIS AND GLUCONEOGENESIS. Biochemistry. 1964 Nov;3:1687–1692. doi: 10.1021/bi00899a015. [DOI] [PubMed] [Google Scholar]


